| disease | Lung Cancer (Surgery) |
| alias | Primary Bronchogenic Carcinoma, Lung Cancer, Bronchogenic Carcinoma |
The incidence and mortality rates of lung cancer are rapidly increasing, which is a global trend. In many developed countries, lung cancer ranks first among common malignant tumors in men and second or third among common malignant tumors in women. Smoking, passive smoking, and environmental pollution—especially air pollution—are the main culprits behind this grim reality, yet these issues remain long-standing and unresolved challenges. Another epidemiological trend is the significant change in the histological types of lung cancer between genders. The proportion of squamous cell carcinoma in men has sharply declined (leading to a corresponding increase in adenocarcinoma), while the incidence of adenocarcinoma continues to rise in women. Lung cancer severely endangers public health and threatens lives, yet the treatment outcomes for lung cancer remain highly unsatisfactory to date.
bubble_chart Clinical Manifestations
The most common pulmonary symptoms, ranked by frequency of occurrence, are: ① Cough, mostly dry cough, without phlegm or with little phlegm, accounting for 67–87% of all symptoms. Cough as the initial symptom occurs in 55%–68.4% of all cases. ② Hemoptysis, occurring in 31.6%–58.5% of cases, is mostly intermittent, with blood streaks or spots in the phlegm, while massive hemoptysis is rare. This serves as the initial symptom in about one-third of cases. The general public tends to take blood in phlegm seriously, making it one of the main reasons prompting patients to seek medical attention. Doctors must exercise caution in diagnosis, with X-rays, sputum cytology, and, when necessary, fiberoptic bronchoscopy being routine examinations that should never be taken lightly. ③ Chest pain occurs in 34.2%–62% of cases, mostly dull pain, with 24% of cases starting with this symptom. If the pain is severe, possibilities such as pleural seeding or rib invasion should be considered. ④ Shortness of breath appears in 10%–50% of cases, with about 6.6% of patients starting with this symptom. In the early stages, it is caused by tumor obstruction of the bronchus leading to atelectasis of a lung segment or lobe. After a short period of adaptation, the shortness of breath may lessen or resolve. If the condition is severe, it may indicate pleural or pericardial effusion, compression of the trachea or carina, or extensive pulmonary metastases, suggesting a late-stage disease course. ⑤ Fever occurs in 6.6%–39% of cases, with 21.2% starting with this symptom. It is often low-grade and caused by tumor obstruction of the bronchus leading to distal segmental, lobar, or even whole-lung atelectasis. If secondary infection occurs, fever may persist. This obstructive pneumonia sometimes appears on X-rays as lobar pneumonia, and anti-inflammatory treatment may temporarily be effective, with the collapsed lung re-expanding, leading to a misdiagnosis of simple pneumonia. However, inflammation often recurs in the same area after some time. Recurrent segmental inflammation in a fixed area of the lung should alert medical staff to the possibility that this inflammation is superficial, caused by the underlying obstruction of the bronchial lumen by a tumor.
Lung cancer patients experiencing severe chest pain, hoarseness, superior vena cava compression syndrome, invasion of the brachial plexus, sympathetic or phrenic nerves causing pain or paralysis, esophageal compression leading to dysphagia, pericardial tamponade, severe bone pain, headache, or liver pain—all these are due to tumor invasion of the respective organs and are symptoms of advanced-stage disease.1. X-ray diagnosis is the most commonly used method for diagnosing {|###|}lung cancer{|###|}, with a positive detection rate of over 90%. It includes various techniques such as fluoroscopy, plain radiography, tomography, chest computed tomography (CT), magnetic resonance imaging (MRI), and bronchography. Due to its high resolution, CT has been rapidly adopted. Previously used methods like bronchography and pulmonary {|###|}stirred pulse{|###|} angiography have gradually been replaced by it. The clinical practice principle is to follow the above order, progressing from simple to complex and from low-cost to high-cost examinations. The widespread use of CT began in the 1970s. It outperforms conventional chest X-rays in identifying lesion locations, their relationship with surrounding organs, small pleural implants or minimal effusions, segmental atelectasis, mediastinal lymph node enlargement, and tiny pulmonary metastatic lesions. However, it has limitations. Enlarged lymph nodes do not necessarily indicate metastasis; inflammatory lymph nodes may exceed 1.5 cm in diameter, while cancerous metastatic nodes can be smaller than 0.5 cm. Therefore, a single enlarged lymph node should be regarded with suspicion but not as a contraindication for surgery. Of course, if the nodes have fused into a mass, metastasis can be confirmed. Conventional chest X-rays display larger lesions (slightly magnified compared to their actual size) and can clearly reveal their density, borders, pleural changes, central liquefaction, and other features. Thus, plain radiography should always be performed first, followed by chest CT if further clarification is needed. Abdominal CT is highly useful for detecting metastases in organs such as the liver, kidneys, and adrenal glands.
In more {|###|}advanced stage lung cancer{|###|}, the following may be observed: large nodular masses in the lung fields or hilum, lobulated in shape, generally uniform in density, with spiculated margins, and sometimes central liquefaction forming thick-walled, eccentric cavities with irregular inner walls. If the tumor obstructs lobar or main bronchi, lobar or whole-lung atelectasis may occur. Pleural involvement can lead to significant pleural effusion, and chest wall invasion may result in rib destruction.
Alveolar cell carcinoma, also known as bronchiolar carcinoma, is relatively rare and more common in women. The solitary type often appears as small infiltrates that grow slowly, making it easy to misdiagnose as {|###|}subcutaneous node{|###|}. However, careful follow-up observation usually reveals persistent shadow enlargement, regardless of how slow the progression is. This remains a crucial diagnostic criterion for {|###|}lung cancer{|###|}. Do not dismiss it due to slow growth. Metastatic tumors or {|###|}foxtail millet{|###|}-sized pulmonary {|###|}subcutaneous nodes{|###|} can be difficult to differentiate.
3. Sputum Exfoliative Cytology Examination Simple and easy to perform, but the positive detection rate is only 50–80%, with a false-positive rate of 1–2%. This method is suitable for diagnosis in high-risk populations. To improve the detection rate, attention must be paid from the very beginning of sputum collection. First, patients should be taught to cough up genuine sputum from the "deep" part of the lungs, not just saliva or oral fluid. If necessary, medication can be used to stimulate sputum production. Second, samples should be selected, smeared, and fixed while the sputum is fresh, followed by staining and slide reading.
4. Percutaneous Lung Biopsy Suitable for peripheral lesions and cases unsuitable for thoracotomy due to various reasons, where other methods fail to establish a histological diagnosis, commonly used in internal medicine. Thoracic surgery, equipped with thoracoscopy and exploratory thoracotomy, uses this method less frequently. Currently, fine needles are preferred for their safety and fewer complications. The positive rate is 74–96% for malignant tumors and lower (50–74%) for benign tumors. Complications include pneumothorax (20–35%, with about 1/4 requiring intervention), minor hemoptysis (3%), fever (1.3%), air embolism (0.5%), and needle tract seeding (0.02%).
5. Mediastinoscopy First performed by Harken et al. in 1954 and further refined by Carlens et al. in 1959, laying the foundation for modern mediastinoscopy. A transverse incision is made at the suprasternal notch, followed by blunt dissection of the anterior cervical soft tissues to reach the pretracheal space. A pretracheal pathway is bluntly dissected, and an endoscope is inserted to slowly pass behind the innominate artery, observing the paratracheal, tracheobronchial angle, and subcarinal regions for enlarged lymph nodes. A fine needle is first used for aspiration to confirm the absence of blood vessels, followed by biopsy forceps to obtain tissue samples. The overall positive rate in large case series is 39%. Some authors report that 25% of cases avoid unnecessary exploration, while others note a false-negative rate of 8%, often due to metastatic lymph nodes outside the scope of mediastinoscopy. The consensus is that mediastinoscopy should be performed when CT shows enlarged lymph nodes in the pretracheal, paratracheal, or subcarinal regions (groups 2, 4, 7). The procedure is performed under general anesthesia, with a mortality rate of 0.04% and a complication rate of 1.2%, including pneumothorax, recurrent laryngeal nerve palsy, bleeding, and fever.
6. Magnetic Resonance Imaging (MRI) A newer imaging technique than CT, MRI provides clearer visualization of central tumors and their relationship with surrounding organs and blood vessels in lung cancer diagnosis and staging. Without requiring contrast agents, MRI leverages the "flow void" phenomenon to clearly depict major vascular anatomy, determining whether tumors invade or encircle blood vessels. If involvement exceeds half the circumference, resection is difficult; if it exceeds three-fourths, surgical exploration is unnecessary. MRI also clearly displays tumor invasion into soft tissues.
7. Bone Scintigraphy or Emission Computed Tomography (ECT) Due to increased blood flow, osteoblastic activity, and heightened metabolism in bone lesions, the bone-seeking agent 99mTc-MDP (methyl diphosphonate) accumulates at the lesion site, detecting metastases 3–6 months earlier than conventional X-rays. If the lesion reaches the intermediate stage (second stage), with decalcification exceeding 30–50%, both X-rays and bone scans show positive findings. If osteoblastic activity is inactive and metabolism is low, the bone scan may be negative while X-rays are positive, complementing each other to improve diagnostic accuracy.
8. Positron Emission Tomography (PET) Using 2-[18F]fluoro-2-deoxy-D-glucose (FDG), whole-body PET can detect unexpected extrathoracic metastases. No false positives occur in extrathoracic metastasis cases, but false positives may arise in mediastinal granulomas or other inflammatory lymph node diseases, requiring cytological or biopsy confirmation. Nonetheless, PET undoubtedly enhances preoperative staging accuracy.
bubble_chart Treatment Measures
Surgical treatment has been recognized as the preferred method for treating lung cancer, and radical resection remains the only treatment that offers the possibility of curing lung cancer patients and restoring them to normal life. Based on years of accumulated analysis of surgical outcomes, the following are the surgical indications for lung cancer:
1. Non-small cell lung cancer at clinical stages I, II, and IIIA, meaning the T-stage is no greater than 3, with tumor invasion limited to the diaphragm, chest wall, pleura, pericardium, or proximity to the carina accompanied by total lung collapse. The upper limit for lymph node involvement is N2, with metastasis to the ipsilateral mediastinum but no further spread. M should be 0, indicating no distant metastasis.
2. The indications for small cell lung cancer are stricter, limited to stages I and II. For intraoperatively confirmed N2 lesions, if radical resection is still achievable, surgical efforts should not be abandoned. Postoperative chemotherapy is mandatory for small cell lung cancer.
3. For pulmonary shadows without cytopathological confirmation, if the clinical history, physical examination, and imaging findings suggest a higher likelihood of malignancy than benign sexually transmitted disease, the patient should be advised to undergo surgical exploration. If the macroscopic appearance during thoracotomy remains inconclusive, rapid pathological or cytological examination can be performed. Our view is that an aggressive approach should be taken for indeterminate pulmonary masses, with early surgical exploration. Intraoperative rapid examination can provide definitive pulse taking and palpation diagnosis and reliable guidance for the extent of resection. Even for benign sexually transmitted disease, local resection removes both the disease and the patient’s psychological burden, which is entirely justified.
4. Even in advanced cases where T reaches stage 4, N reaches stage 3, or M is 1 (e.g., isolated brain metastasis), palliative surgery may still be performed to alleviate symptoms in cases of uncontrolled pulmonary complications, persistent high fever, or lung collapse severely impairing gas exchange and causing hypoxemia. This, however, is an exceptional last resort.
Contraindications for Surgery
The surgical indications for lung cancer have been outlined above. In short, contraindications refer to cases beyond these indications, such as various T4 tumors invading the mediastinum, heart, major vessels, trachea, esophagus, vertebral body, carina, or additional nodules in the same lobe, or the presence of malignant pleural effusion. N-stage reaching 3, with metastasis to contralateral hilar, mediastinal, supraclavicular, or axillary lymph nodes. Distant metastasis to organs such as the liver, bones, brain, or adrenal glands, with M being 1. Patients with severe comorbidities, such as chronic pulmonary infections, lung qi swelling, impaired ventilation and gas exchange, cardiac insufficiency, heart failure, a history of colicky pain or myocardial infarction within the past three months, or a recent cerebrovascular accident.
Preoperative Preparation
Once the physician diagnoses the likelihood of lung cancer and recommends surgical treatment, and the patient accepts this recommendation, the critical preoperative preparation phase begins.
Respiratory Care
Most lung cancer patients are elderly, with varying degrees of chronic bronchitis, lung qi swelling, or other complications due to long-term smoking. Therefore, persuading the patient to quit smoking is of paramount importance. Typically, explaining the risks and linking them to surgical outcomes will encourage patient cooperation and complete cessation. For patients with chronic bronchitis, yellow sputum production, or partial lung collapse or obstructive pneumonia due to tumor obstruction, early antibiotic therapy targeting the causative pathogens based on sensitivity testing is essential to control pulmonary inflammation preoperatively, keeping the temperature below 37.5°C. In addition to systemic antibiotics, localized treatment with nebulized medications can also yield excellent results. For patients with pulmonary subcutaneous node infections, a two-week course of adequate combined anti-tuberculosis therapy should be administered preoperatively to prevent postoperative reactivation or dissemination of subcutaneous node infections due to compromised immune function and lack of anti-tuberculosis protection.
Psychological care
To enhance cardiac function, an energy mixture (glucose, insulin, potassium chloride, vitamin C, coenzyme A, creatinine, etc.) can be appropriately administered before surgery to protect the myocardium. If there are water-electrolyte imbalances, they should be corrected. For patients with arrhythmias, treatment should be tailored based on the type of arrhythmia: atrial or supraventricular arrhythmias should first be treated with Digitalis-like drugs. If the effect is not significant, quinidine or verapamil can be used instead. Ventricular premature beats should be treated with lidocaine. Depending on the situation, nitroglycerin-like drugs or Salvia can be added to dilate the coronary arteries, and oxygen therapy should also be provided. To improve cardiopulmonary function, patients can be guided to perform stair-climbing exercises, where they climb stairs at a moderate pace, gradually increasing the load from a small amount. Generally, if a patient can climb three flights of stairs without stopping, with post-exercise respiration not exceeding 20/min and heart rate not exceeding 100/min, the patient can likely tolerate a lobectomy.
Measurement of pulmonary ventilation function
The following indicators are contraindications for surgery or require careful consideration: ① Maximum ventilation volume less than 50% of the predicted value; ② Forced expiratory volume in the first second (FEV1) <1L;③血氣分析PO2 <9.3kPa。當FEV1 >2.5L indicates the patient can likely withstand a pneumonectomy. If FEV 1 is between 1–2.4L, surgery should be carefully considered.
Selection and evaluation of surgical procedures for lung cancer treatment
The history of lung resection for lung cancer began with pneumonectomy. Later, with advancements in surgical techniques and improvements in anesthesia methods, lobectomy gradually replaced pneumonectomy as the standard procedure for lung cancer treatment. Since Allison pioneered right upper lobectomy with sleeve resection for lung cancer in 1952, the rate of pneumonectomy has rapidly declined. Lobectomy now accounts for 70%, while pneumonectomy has decreased to 20%. If sleeve lobectomy is included under lobectomy, the rate of lobectomy rises to nearly 80%, and in some hospitals, it can reach 85%. This shift has been widely accepted and implemented by thoracic oncologic surgeons without increasing postoperative complication or mortality rates (Table 1). This type of reconstructive surgery embodies two fundamental principles of lung cancer surgical treatment: first, preserving as much lung tissue as possible. Upon closer examination, these two principles have a priority order. If both cannot be achieved simultaneously, the first principle should be prioritized over the second, provided the patient's cardiopulmonary function can tolerate it. Specifically, if lobectomy (including bilobectomy or sleeve resection) cannot achieve radical treatment, sacrificing some functional lung tissue may be necessary. However, another tendency should be avoided: performing sleeve lobectomy to test surgical skills when conventional lobectomy could eradicate the tumor, thereby increasing surgical time and the risk of postoperative complications. The key steps of sleeve lobectomy are standard lobectomy plus bronchial resection and reconstruction, which is not overly technically challenging and can generally be performed by well-trained thoracic surgeons. If intraoperative findings reveal tumor invasion into mediastinal organs or extensive mediastinal lymph node metastasis beyond a certain level, making radical resection impossible, the rationality of surgical resection should be judged based on the tumor's condition. If there is atelectasis, secondary obstructive inflammation, or lung abscess where antibiotics alone cannot control the infection, palliative resection may be performed to alleviate symptoms.
Table 1: Rates of pneumonectomy versus lobectomy (partial domestic data)
| Author | Year | Number of cases | Pneumonectomy % | Lobectomy% |
| Sun Chengfu et al. | 1980 | 96 | 30.2 | 69.8 |
| Li Chunling et al. | 1981 | 125 | 21.6 | 78.4 |
| Huang Shaokui et al. | 1981 | 75 | 18.7 | 77.3 |
| Huang Guojun et al. | 1985 | 601 | 12.3 | △74.0 |
△If combined with the 11.2% from sleeve lobectomy, this value should be 85.2%.
When the patient is elderly or has a history of contralateral pneumonectomy with compromised cardiopulmonary function, reaching the critical threshold for thoracotomy exploration, a partial lung resection or lung-sparing resection—namely, selective segmentectomy or wedge resection—can be performed if the lesion is peripheral and no larger than 5 cm in diameter. According to Jensik et al., among 168 cases of segmentectomy, 16 (35.6%) experienced local recurrence in the operated lobe or mediastinum within 5 years. Mc Cormack reported 61 cases of lung-sparing resection, including 35 Stage I cases with a 5-year survival rate of 33%, but the local recurrence rate in this group was 19.3%.
For T3 tumors in Stage IIIA, a cautious approach with special considerations is warranted. If the tumor has invaded the chest wall, en bloc resection of the affected lobe and involved chest wall should be performed, with the chest wall defect repaired using materials like Marlex mesh or Dacron. Martini and Mc Cormack reported 155 cases of chest wall tumor resection, including 63 cases of lung cancer and 92 cases of metastatic fleshy tumors or carcinomas, with an overall 5-year survival rate of 20%.
For T3 tumors near the carina, carinal resection and reconstruction combined with lung resection can be performed. Two domestic groups reported surgical mortality rates of 6.3% and 15% (3/20), with one group achieving 5-year survival in only 2 cases (out of 31) and the other reporting a 5-year survival rate of 20% (1/5). However, the latter group included T4 cases where the tumor had invaded the carina or trachea.
As mentioned earlier, T4 cases are generally considered contraindications for surgery. However, some Japanese scholars have reported extended resections of lung cancer and adjacent organs, with 5-year survival rates ranging from 10% to 26%. However, no long-term survival was observed when partial left atrium, superior vena cava, pulmonary artery, esophagus, or diaphragm were resected, with most patients dying within 2 years postoperatively. If multiple organs were resected, the outcomes were even worse, with all patients dying within 6 months.
Patients with malignant pleural effusion and T4 are classified as having pleural seeding and are considered contraindicated for surgery. Shanghai Chest Hospital reported 31 cases of total pneumonectomy and pleurectomy combined with local liquid nitrogen cryotherapy, which alleviated symptoms, but only 3 patients survived for 2 years. Besides surgical methods like pleurectomy, other approaches to control malignant pleural effusion include: ① Closed drainage combined with sclerosing agents, such as tetracycline 7–20mg/kg dissolved in 10ml of saline administered weekly, or quinacrine 100–200mg per dose, or talc powder; ② Mitomycin 20mg per dose, administered weekly; doxorubicin 40mg per dose, administered every 3 weeks; cisplatin 80mg per dose, administered every 4 weeks; ③ Intrapleural application of immunostimulants such as BCG cell wall skeleton 100mg per dose, administered weekly. If pleural effusion decreases, it can be switched to intramuscular injection. OK-432, prepared from Streptococcus pyogenes, can also be used. For stage IIIA cases with N2, preoperative examinations should be conducted as thoroughly as possible to ensure accurate staging. Based on the experience of our hospital and Shanghai Chest Hospital, if the T stage is 1, the 5-year survival rates post-surgery are 33%, 27.7%, and 21%, respectively. If the T stage is 3, the 5-year survival rate drops to 0% and 14.4%. If preoperative examinations fail to detect N2 but it is confirmed during intraoperative exploration, the authors recommend cautious management for such cases. When metastatic N2 involves only one or two levels, the metastasis is confined to the lymph nodes without extending to the surrounding tissues, and the T stage does not exceed 2, a radical pneumonectomy with mediastinal lymph node dissection should be performed to achieve a favorable long-term outcome.
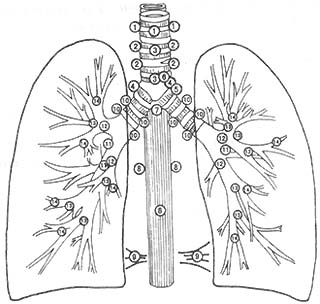
The lymphatic drainage of the lungs follows certain patterns. The upper lobe of the right lung drains to the right hilum and the right upper mediastinal lymph nodes. The middle lobe of the right lung drains to the middle and lower lobe confluence area lymph nodes, subcarinal nodes, and right upper mediastinal nodes. The lower lobe of the right lung drains to the middle and lower lobe confluence area, subcarinal nodes, inferior pulmonary ligament nodes, and right upper mediastinal lymph nodes. The upper lobe of the left lung drains to the subaortic (Botallo) lymph nodes and the left anterior upper mediastinal lymph nodes. The lower lobe of the left lung drains to the upper mediastinal lymph nodes. Using Naruke's lymph node map (Figure 1), the lymph node metastasis (N status) of lung cancer can be illustrated (Table 2).
Table 2 Relationship between the site of lung cancer and the location of metastatic lymph nodes
| Lung Site | Common Metastatic Mediastinal Lymph Nodes | |
| Right | Upper Lobe | 2R, 3, 4R |
| Middle Lobe | 2R, 4R, 7, 9 | |
| Lower Lobe | 7, 8, 9 | |
| Left | Upper Lobe | 5L, 6L |
| Lower Lobe | 7, 8, 9, and 2R, 4R | |
During surgery, lymph nodes in the relevant drainage areas should be thoroughly dissected based on the location of the lung cancer and its lymphatic drainage. Due to the rich anastomoses of mediastinal lymphatic vessels, skip metastases, retrograde metastases, and even contralateral mediastinal metastases (right-sided lesions metastasizing to the left mediastinum) can occur, which should be comprehensively considered during diagnosis and treatment. In fact, when there is extensive lymph node metastasis in the hilum and/or mediastinum, attempting to achieve radical resection with a scalpel is often an unrealistic ideal.
|
|
|
|
|
|
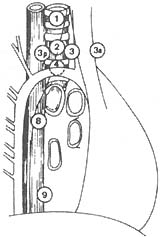
Figure 1 Anatomical numbering of pulmonary and mediastinal lymph nodes (Naruke)
1. Superior or highest mediastinal; 2. Paratracheal; 3. Pretracheal; 3a. Anterior mediastinal; 3b. Retrotracheal or posterior mediastinal; 4. Tracheobronchial; 5. Subaortic or Botallo's; 6. Para-aortic (ascending aorta); 7. Subcarinal; 8. Paraesophageal (below carina); 9. Pulmonary ligament; 10. Hilar; 11. Interlobar; 12. Lobar—upper lobe, middle lobe, lower lobe; 13. Segmental; 14. Subsegmental
Key points of surgical technique
The success of surgical treatment for lung cancer primarily relies on meticulous perioperative management and the surgeon's proficient skills. Based on a thorough understanding of the anatomy of the pulmonary hilum, bronchi, and blood vessels, gentle and precise sharp dissection is employed, followed by proper ligation, suturing, and division of the relevant blood vessels and bronchi. The principles of the procedure are as follows:
1. Comprehensive exploration and understanding of the surgical tumor situation. In addition to the characteristics and location of the mass and the degree of external invasion, it is also necessary to assess whether lymph nodes in the hilar and mediastinal areas are enlarged or fused into clusters. To determine the presence of metastasis, frozen biopsy of the mass and/or lymph nodes may be performed if necessary. If there is pleural effusion or suspicious nodules in other pulmonary or pleural membranes, frozen biopsy should also be prioritized to rule out the possibility of tumor spread, i.e., to exclude the condition from reaching stage IIIB or IV, which would render it unsuitable for surgical resection.
2. Surgical manipulation must be gentle. Avoid squeezing or kneading the tumor to prevent iatrogenic hematogenous dissemination.
3. Principle of ligating veins first. Practice has shown that the previously advocated practice of ligating and cutting the pulmonary vein first to prevent cancer cells from entering the systemic circulation and spreading throughout the body was overly cautious. The long-term outcomes of cases where the vein was ligated first showed no difference from those where the pulmonary artery was ligated first. The latter approach also avoids the drawback of blood stasis in the resected lung tissue.
4. Handling of vascular branches. Do not rigidly adhere to the number of pulmonary artery branches described in anatomy textbooks, as individual variations are significant. Surgeons must not assume that ligating the number of vascular branches described in textbooks is sufficient. The only indication that all branches have been ligated is the retraction of the main vessel to the root of the hilum. Additionally, be aware that extremely fine branches may exist, and slight negligence during dissection can lead to bleeding.
5. Anomalies of pulmonary veins. Some patients lack the inferior pulmonary vein, with pulmonary venous return relying solely on the superior pulmonary vein. The exact incidence is unknown, but this anomaly is more common in the left lung. Therefore, during left upper lobectomy, the presence of the inferior pulmonary vein must be confirmed to avoid ligating and cutting the only pulmonary vein, which would leave the remaining lung tissue without venous drainage, leading to severe congestion, hepatization, and persistent hemoptysis, necessitating a second surgery for resection.
6. Residual cancer at the bronchial stump. Among 122 N2 cases in the Thoracic Surgery Department of the Cancer Hospital of the Chinese Academy of Medical Sciences, 3 had positive stumps, none of whom survived for 3 years. In a group of 135 sleeve lobectomy cases, postoperative examination revealed incomplete margins in 3 cases, with distances from the tumor being 1.5 cm, 2 cm, and 2 cm, respectively. Follow-up also identified 5 cases of anastomotic recurrence. Since pathological examinations cannot always perform serial sections, the actual rate of residual cancer at the stump is likely higher than reported. These facts suggest that the margin distance from the tumor should ideally exceed 2 cm. 2 <1cm,如肉眼判斷有懷疑時,應即刻送切緣的冰凍切片,若確有殘餘,應進一步截除之長度。在肺功能可以承受的前提下,甚至施行全肺切除術,力爭達到根治的目的。
7. Sleeve lobectomy. Taking the most common right upper lobe sleeve resection as an example, after thoracotomy, the tumor's location, size, and extent of invasion are first assessed. If the tumor is confirmed to be at the lobar orifice without invasion of the main or intermediate bronchus and no N2 lymph nodes are found in the mediastinal dissection, sleeve resection can be considered anatomically and oncologically appropriate. The Cancer Hospital of the Chinese Academy of Medical Sciences' experience shows that in cases with N2 (pathological stage IIIA), the 5-year survival rate is 16.6%, significantly lower than the 62.5% for stages I and II. The hilum is dissected, and the corresponding pulmonary artery and vein branches are ligated and cut. The lobar bronchial orifice and adjacent right main bronchus are freed. To address the differing cutting methods for the two stumps, the main bronchus should be cut vertically, while the intermediate bronchus is cut obliquely. Typically, tumors at the lobar orifice invade the lateral wall of the main or intermediate bronchus, while the medial wall remains normal. An oblique cut from the outside inward preserves more of the medial wall, allowing the lower stump's diameter to approximate that of the upper stump. 2 2
During the anastomosis, fine absorbable synthetic sutures should be used. First, interrupted or everted mattress sutures are applied to the membranous portion on the side. Since this area is farthest from the surgeon and only becomes visible when the lateral part is open, it must be sutured first. Additionally, if a fistula occurs in this area after the anastomosis is completed, repair is nearly impossible. As the procedure is performed under direct vision, the suturing must be precise and airtight to ensure a successful anastomosis on the first attempt. The suture spacing should be approximately 2mm, with knots tied outside the lumen, ensuring appropriate tension and alignment. Next, the anterior side is sutured. To address any discrepancy in the diameter of the two ends, the suture spacing can be adjusted during the anastomosis: the side with the larger diameter should have slightly wider spacing, while the side with the smaller diameter should have appropriately reduced spacing. This ensures that despite the size difference, both sides are sutured securely without fistula formation. If any air leakage (fistula) is detected, it should be carefully sutured. If a fistula is suspected at the posterior anastomotic site, repair is often extremely difficult due to the obscured view and challenging access for the surgeon. In such cases, it may be more effective to remove a few sutures from the anterior side to expose the posterior defect for proper repair. If difficulties persist, the entire anastomosis may need to be redone. To reinforce the anastomosis, a pedicled pleural flap or pericardial flap can be wrapped around the anastomotic site. This measure also helps prevent severe complications such as massive hemorrhage from a pulmonary stirred pulse fistula. Data from 2004 cases in the Thoracic Surgery Department of the Cancer Hospital of the Chinese Academy of Medical Sciences showed that among 155 cases of sleeve lobectomy, the 5-year survival rate was 53.9% (57.3% for radical resection and 14.3% for palliative resection), significantly better than the 31.2% rate for pneumonectomy. Possible reasons for this include: 1. Early-stage (Stage III) cases accounted for 89.2% of the sleeve group. 2. The pathological type in the sleeve group was predominantly squamous cell carcinoma (72.9%). 3. Preserving more functional lung tissue likely contributed to better postoperative quality of life for these patients.
When a tumor at the bronchial orifice simultaneously invades or adheres to the pulmonary artery, a pulmonary artery plasty can be performed simultaneously with the seasonal epidemic. If the affected area is less than one-third of the circumference of the pulmonary artery, a lateral wall resection can be done. If it exceeds one-third of the circumference, a segmental resection with end-to-end anastomosis should be performed. To avoid tension at the anastomosis site, the length of the pulmonary artery resection should preferably not exceed 3 cm. The distance between the vascular margin and the tumor should not be <0.5cm。同時行左上葉袖式切除時,肺動脈幹切除長度可達5cm。一般先作支氣管成形術,再吻合血管。術後需加抗凝治療。隆突成形肺切除之注意點是氣管支氣管吻合部無張力,因此切除長度之安全範圍從氣管下端到對側支氣管不超過4cm。更具體點說,病變同側主運載氣管內延伸距隆突不>1 cm. For lesions in the lower trachea, the distance from the carina should not exceed 2 cm, and for lesions in the contralateral main bronchus, it should not exceed 1.5 cm, which are indications for carinal pneumonectomy.
The outcomes of surgical treatment for lung cancer in our country have remained relatively stable over the past 20 years, similar to international trends. Among 6 groups totaling 8,960 cases, the resection rate was 80.4% to 91.4%. The postoperative complication rate ranged from 1.7% to 22.3%, and the postoperative mortality rate (within 30 days) was 0.8% to 3.1%. The 5-year survival rate after resection was 27.2% to 42%. Lobectomy accounted for 70.3%, pneumonectomy for 20%, sleeve lobectomy for 4%, partial lung resection for 5.1%, and carinal resection with reconstruction for 0.6%. In terms of pathological types, squamous cell carcinoma was the most common, accounting for 47.5% of the 8,960 cases, followed by adenocarcinoma at 32.6%. Next in order were small cell lung cancer at 10.3%, large cell lung cancer at 3.3%, and others at 5.8%. The complication rates and surgical mortality (within 30 days) for different surgical approaches had several characteristics. According to an analysis of 1,721 resection cases from the Thoracic Surgery Department of Liaoning Cancer Hospital, the highest complication rate was observed in the partial resection group, which involved the least amount of lung tissue removal. Although sleeve lobectomy is technically more complex, it did not show an increase in complications. One possible reason for analysis is that wedge or segmental resections often create more raw surfaces. If hemostasis is incomplete or small bronchial stumps are not properly sutured and ligated, the chances of postoperative hemothorax or pneumothorax increase. However, in terms of postoperative mortality, pneumonectomy, due to the loss of the most functional tissue and the greatest negative impact on circulatory and respiratory functions, had the highest mortality rate among all surgical approaches.
In the surgical treatment of lung cancer, various complications are inevitable. As mentioned at the beginning of this section, the reported incidence rates vary widely among different studies, with large-scale domestic data ranging from 1.7% to 22.3%. In the thoracic surgery department of the Cancer Hospital of the Chinese Academy of Medical Sciences, among 1,721 resection cases out of 2,004 total cases, 271 complications occurred, yielding an overall incidence rate of 15.7%. When the results were divided into three 10-year periods, a clear downward trend in complications was observed: the complication rate was 25.6% in the 1960s, decreased to 19.2% in the 1970s, and further dropped to 14.3% in the 1980s. The most common complications were pulmonary issues, including atelectasis, pneumonia, and respiratory failure, totaling 57 cases (21% of all complications). Among the 16 cases of respiratory failure, 7 resulted in death, yielding a mortality rate of 43.8%. The second most frequent were cardiac complications, totaling 30 cases (11.1% of all complications). The third most common were bronchopleural fistula and intrathoracic hemorrhage, each occurring 16 times (5.9% of all cases). Among the intrathoracic hemorrhage cases, 2 resulted in death (12.6% mortality). Additionally, there were 10 cases of empyema (3.7%), 4 cases of pulmonary embolism, and 132 miscellaneous complications. It is evident that respiratory failure, arrhythmia, intrathoracic hemorrhage, and pulmonary embolism are the four complications with high mortality rates, classified as severe complications. These should be prioritized for prevention, and aggressive rescue measures must be taken if they occur.
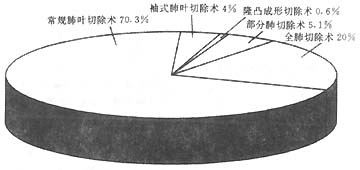
Figure 2 Schematic diagram of 4 groups of 2884 domestic lung cancer resection surgeries
Comprehensive treatment of lung cancer
1. Preoperative radiotherapy: Its theoretical basis includes: ① Eliminating subclinical lesions outside the surgical area, such as microscopic metastases in the mediastinum; ② Reducing tumor volume and infiltration into adjacent tissues, thereby increasing the anatomical planes for dissection; ③ Weakening tumor cell viability, reducing the likelihood of local implantation and distant metastasis. The anticipated benefits are improved resection rates and long-term survival rates. However, clinical practice results have been contrary to expectations, with neither of these goals being achieved. Therefore, it can be said that preoperative radiotherapy combined with surgery has not benefited patients and is no longer routinely adopted in clinical practice.
2. Intraoperative radiotherapy: The implantation of medical radioactive isotopes (125I, 222Rn) into unresectable tumors during thoracotomy has yielded satisfactory therapeutic outcomes, as reported by physicians such as Hilaris BS from the Sloan Kettering Memorial Hospital in the U.S. In a group of 105 cases, the mortality rate was 5% (52/105). A comparison of the two isotopes showed that 125I had better tumor shadow disappearance rates and local control rates than 222Rn. The results of a randomized trial involving 9 groups totaling 2128 cases of this intraoperative radiotherapy combination indicated that postoperative radiotherapy significantly harms survival rates, with a hazard ratio of 1.21 (95% confidence interval 1.08–1.34). This 21% relative increase in mortality risk corresponds to a 7% reduction in the 2-year survival rate, from 55% to 48%. This detrimental effect is particularly pronounced in stage I–II N0N1 cases. In stage III N2 cases, the destructive effect is less evident. The report concludes that postoperative radiotherapy is harmful rather than beneficial to the survival rates of radically resected stage I–II non-small cell lung cancer and thus should not be routinely adopted. The report also mentions that radiation dose and planning do not affect outcomes, meaning there is currently no evidence that any particular protocol is less harmful than others. The authors suggest that future trials should focus only on stage III N2 cases, as the role of postoperative radiotherapy in these advanced-stage cases remains undetermined. Repeating the same trials in early-stage non-small cell lung cancer resection cases is no longer meaningful.
Surgery-assisted preoperative and postoperative chemotherapy
1. Preoperative Adjuvant Chemotherapy In the 1970s, the first application of chemotherapy for early-stage solid tumors, specifically germ cell tumors, achieved remarkable efficacy. The combination of chemotherapy followed by surgery to remove residual lesions significantly improved survival rates. This success inspired oncologists to adapt the treatment model for germ cell tumors to other solid tumors. After multi-drug regimens proved effective for both non-small cell and small cell lung cancer, clinical trials for the so-called "neoadjuvant" approach rapidly emerged. The earliest neoadjuvant protocol was ethically tested by a Toronto group on a small number of small cell lung cancer cases. This involved multi-drug preoperative chemotherapy followed by surgery and postoperative consolidative radiotherapy. Retrospective comparisons showed that this combined therapy could improve survival rates for very early-stage small cell lung cancer. The U.S. Lung Cancer Study Group and other medical centers applied this classical formula to non-small cell lung cancer, using multi-drug preoperative chemotherapy containing platinum compounds along with preoperative radiotherapy, targeting stage IIIA cases. Retrospective comparisons demonstrated improved median survival and overall survival rates. With the advent of more effective drugs, the response rate to single-agent induction chemotherapy reached 70%, with a complete response (CR) rate of 10%. Retrospective analyses found that cases showing a response and downstaging had improved survival rates compared to historical single-modality surgical cases of the same stage. Randomized phase III clinical trials using induction chemotherapy as the experimental arm have yielded results in three small-scale studies, showing significantly better outcomes compared to surgery-alone groups.
One of the unresolved issues is whether induction chemotherapy combined with surgery is effective in early-stage clinical IB (T2N0M0) and stage II (T1N1M0, T2N1M0, and T3N0M0). Secondly, the optimal number of cycles for induction chemotherapy remains undetermined. What is certain is that only cases responsive to chemotherapy, with a downstaged (earlier) clinical TNM classification, can benefit. If the optimistic results of multi-drug preoperative induction are confirmed in well-designed prospective clinical trials, most lung cancer cases still amenable to radical resection will receive this multidisciplinary comprehensive treatment in the future.
2. Postoperative Adjuvant Chemotherapy Attempts at adjuvant monotherapy after radical resection of lung cancer have proven ineffective. The drug groups (cyclophosphamide, methotrexate) and the control group showed similar 5-year survival rates (cyclophosphamide group 24.9%, cyclophosphamide + methotrexate group 25.7%, control group 23.5%). Subsequent use of the CAMP and CAP combination regimens demonstrated prolonged disease-free survival after surgery in stage II and III non-small cell lung cancer. The question yet to be answered is whether postoperative adjuvant chemotherapy has any beneficial effect following induction chemotherapy.
The symptoms of lung cancer lack specificity and are relatively complex. As mentioned earlier, its imaging findings resemble those of some common pulmonary diseases such as pulmonary tuberculosis, bronchopneumonia, and lung abscess. In fact, it can also cause secondary obstructive inflammation, pulmonary suppuration, and atelectasis, resulting in a high misdiagnosis rate. The consequence of misdiagnosis is that a significant number of patients lose the opportunity for radical treatment. This requires professionals not only to be familiar with the pathological changes and corresponding clinical manifestations of lung cancer at various stages of development but also to master the pathology and clinical presentations of common pulmonary diseases. They must discern the truth from falsehood among observations that appear similar but actually differ in dominant and subordinate qi, and make accurate differential diagnoses.
(1) Tuberculosis
1. Spherical pulmonary tuberculosis (tuberculoma) is the most common pulmonary disease that needs to be differentiated from lung cancer. Reports indicate that in a tuberculosis hospital, 45% of 460 lung cancer cases were misdiagnosed as pulmonary tuberculosis. Among various types of pulmonary tuberculosis, spherical tuberculous lesions (commonly called tuberculoma) are most easily confused with round peripheral lung cancer. Tuberculous spherical lesions are more common in young people under 40 years old, rarely present with bloody sputum, show little change in erythrocyte sedimentation rate, and 16–28% of patients have tuberculosis bacteria detected in their sputum. Peripheral lung cancer is more common in patients over 40 years old, often presents with bloody sputum, and 40–50% of cases have positive cancer cells in sputum. In imaging, tuberculous spherical lesions are mostly round, located in the apical or posterior segments of the upper lobe, small in size (generally no more than 5 cm in diameter), with smooth borders and uneven density, sometimes showing calcification. In 16–32% of cases, bronchial shadows draining toward the hilum can be seen, and pleural retraction is rare. Growth is slow, and if central liquefaction occurs, the cavity is usually thin-walled with smooth inner edges. Peripheral lung cancer shows little difference in distribution between the upper and lower lobes (upper:lower ratio of 1.8–3:1), often appears nodular, with spiculation and pleural retraction, and grows relatively quickly.
2. Infiltrative pulmonary tuberculosis is generally easier to differentiate from lung cancer. However, in some peripheral adenocarcinoma cases, early lesions are small and manifest as small patches of infiltration or streaks, especially in isolated alveolar cell carcinoma, which can grow very slowly. Initially, it is difficult to distinguish from tuberculosis. Additionally, early X-ray findings of lung cancer may only show segmental atelectasis or grade I obstructive inflammation, which can also be easily confused with tuberculosis. Repeated sputum cytology tests and trial anti-tuberculosis therapy should be conducted, with close follow-up to monitor lesion changes, and systematic and dynamic analysis should be performed.
3. Hilar lymph node tuberculosis is most commonly found beside the trachea in the right upper mediastinum. When inflammation causes swelling and fusion into a mass, it needs to be differentiated from central lung cancer and mediastinal lymph node metastasis. The latter often presents with hemoptysis and atelectasis.
4. Miliary pulmonary tuberculosis is difficult to distinguish from diffuse alveolar cell carcinoma on imaging. Generally, miliary tuberculosis presents with more severe systemic toxic symptoms, which can gradually improve with anti-tuberculosis therapy. Diffuse alveolar cell carcinoma more easily yields cancer cells in sputum.
5. Coexistence of pulmonary tuberculosis and lung cancer: In China, where pulmonary tuberculosis is prevalent, the coexistence of tuberculosis and lung cancer is not uncommon. During tuberculosis treatment, if some lesions improve while others continue to grow and worsen, the possibility of coexisting diseases should be highly suspected.
(2) Infectious lung diseases
1. Pneumonia: Sometimes, lung cancer obstructs the bronchus, causing distal obstructive pneumonia, which can be difficult to distinguish from simple pneumonia. If inflammation in the atelectatic lung tissue worsens, even progressing to lung abscess, the patient may present with fever, cough, yellow sputum, or massive hemoptysis, resembling simple lung abscess. If recurrent inflammation occurs in the same lung area, a tumor-induced obstruction should be highly suspected. If imaging confirms bronchial stenosis or truncation, a definitive diagnosis can be made.
2. Middle Lobe Syndrome refers to middle lobe atelectasis with chronic inflammation or accompanied by bronchiectasis. The cause of atelectasis is the compression of the middle lobe bronchus by enlarged inflammatory lymph nodes around it, leading to luminal narrowing and ventilation obstruction. When the inflammation is controlled and the lymph nodes shrink, no longer compressing the bronchus to restore ventilation, the collapsed alveoli fail to re-expand. Recurrent inflammation in the atelectatic lung leads to intermittent symptoms such as fever, coughing up yellow sputum, or hemoptysis. This condition is generally more common in young individuals with a long history. If the patient is elderly with a short history and nodular shadows are observed in the hilar region, lung cancer should be suspected. Further examinations such as FOB are required for definitive diagnosis.
In summary, the distinguishing features of solitary benign sexually transmitted disease lesions in the lungs are: ① no increase in lesion size for 2 years; ② presence of benign calcification within the lesion.