| disease | Aortic Valve Disease |
Congenital aortic valve disease may not present obvious symptoms in early childhood. Common symptoms of aortic valve disease typically include palpitations, shortness of breath, and angina after exertion. In cases of grade III aortic stenosis or aortic regurgitation, angina is particularly severe due to severe coronary artery blood supply insufficiency.
bubble_chart Etiology
The main conditions requiring surgical treatment for aortic valve disease include the following four types:
(1) **Congenital Aortic Valve Disease** The more common form is bicuspid valve malformation, with clinical manifestations primarily of aortic stenosis. The systolic transvalvular pressure gradient often exceeds 13.3 kPa (100 mmHg). Electrocardiogram findings typically show left ventricular high voltage, often accompanied by strain. X-ray angiography and ultrasound examinations often reveal a smaller left ventricular cavity with concentric myocardial hypertrophy. Severe aortic stenosis may lead to relative mitral regurgitation due to excessive left ventricular systolic pressure.
Another common congenital aortic valve lesion is aortic valve prolapse causing aortic regurgitation. This malformation often occurs in cases of large high ventricular septal defects or aortic sinus aneurysms rupturing into the right ventricle. In cases of large high ventricular septal defects, the corresponding valve leaflet loses the support of the ventricular septum, causing the leaflet to prolapse into the right ventricle during diastole. In cases of ruptured sinus of Valsalva aneurysms, the corresponding aortic valve leaflet prolapses into the left ventricle.
(2) **Rheumatic Mitral Valve Disease with Aortic Valve Involvement** Approximately 20% of rheumatic mitral valve disease cases are complicated by aortic valve lesions. In rheumatic heart disease, isolated aortic valve disease is rare. All three aortic valve leaflets exhibit fibrosis, thickening, contraction, sclerosis, and even calcification, with severely restricted mobility. Therefore, rheumatic aortic valve disease often presents as a combined lesion of stenosis and regurgitation, with a prolonged course and significant impairment of cardiac function.
(3) **Degenerative Changes of the Aortic Valve** The aortic valve leaflets undergo mucoid degeneration, becoming thin and translucent, unable to withstand diastolic aortic pressure, leading to regurgitation. This is commonly seen in syphilitic aortitis, Marfan syndrome, aortic medial necrosis, senile degenerative changes, and ascending aortic aneurysms caused by other factors. Due to severe aortic regurgitation, the pulse pressure of peripheral arteries widens significantly. Hemodynamically, the left ventricle experiences a grade III volume overload, resulting in left ventricular enlargement to the left, downward, and backward. Left ventriculography and ultrasound examinations show marked enlargement of the left ventricular cavity and grade III aortic regurgitation. Contrast medium flows back and forth between the left ventricle and ascending aorta, remaining for an extended period without rapid clearance.(4) **Bacterial Endocarditis-Induced Aortic Valve Disease** Bacterial endocarditis often destroys aortic valve leaflet tissue, causing vegetations, perforations, or tears. Clinically, aortic valve disease caused by bacterial endocarditis typically manifests as aortic regurgitation. Due to the short disease course and abrupt hemodynamic changes, the left ventricle struggles to tolerate the sudden increase in volume load. Additionally, vegetations may detach, leading to systemic arterial embolism.
bubble_chart Clinical Manifestations
Congenital aortic valve disease may present no obvious symptoms in early childhood. Common symptoms of aortic valve disease generally include palpitations, shortness of breath, and angina after exertion. In cases of grade III aortic stenosis or aortic regurgitation, angina is particularly severe due to significant insufficiency of coronary blood supply. Grade I to grade II aortic stenosis or regurgitation may also be entirely asymptomatic. In cases of pure aortic stenosis, a rough systolic murmur can be heard over the aortic valve area, radiating to the neck. In cases of combined aortic valve lesions or grade III regurgitation, in addition to the typical murmur heard over the aortic valve area, a louder to-and-fro blowing murmur can be heard at the second aortic valve area along the left sternal border. The systolic component radiates to the neck via the second intercostal space along the right sternal border, while the diastolic component radiates downward along the left sternal border toward the cardiac apex.
Chest X-ray reveals varying degrees of left ventricular enlargement and dilation of the ascending aorta. In grade III aortic regurgitation, the left ventricle is most notably enlarged to the left, downward, and posteriorly. In aortic stenosis, the left ventricle exhibits concentric hypertrophy. Retrograde aortography can confirm the degree of regurgitation. In sequential imaging, if the regurgitated contrast agent forms only a small triangular shadow below the aortic valve orifice, it is classified as grade I regurgitation; if the contrast agent appears as a long quadrilateral shape extending to the cardiac apex, it is grade II; and if the contrast agent fills the entire left ventricle, it is grade III. Selective left ventriculography can demonstrate the size of the left ventricular cavity and its contractile function. In cases of aortic stenosis, retrograde catheterization often fails to enter the left ventricle. Sequential imaging in retrograde aortography may also show a doming appearance of the aortic valve during ventricular systole, which is a typical feature of aortic stenosis. During retrograde left heart catheterization, significant changes in left ventricular and ascending aortic pressures are observed. In aortic stenosis, left ventricular systolic pressure is markedly elevated, with a transvalvular pressure gradient exceeding 2.67 kPa (20 mmHg) during systole. In aortic regurgitation, aortic diastolic pressure decreases, pulse pressure exceeds 6.67 kPa (50 mmHg), and left ventricular end-diastolic pressure rises.
Echocardiography not only measures the aortic valve orifice size, degree of regurgitation, ascending aortic diameter, and annulus diameter but also determines left ventricular end-systolic and end-diastolic dimensions, providing further insight into left ventricular ejection function. In recent years, the development of color Doppler echocardiography has enhanced the accuracy of this non-invasive examination. Due to its safety, painlessness, and patient acceptability, echocardiography has largely replaced most retrograde aortography procedures.
In cases of congenital aortic valve malformation with no obvious clinical symptoms during early childhood, surgery can be postponed until the child grows older. For congenital aortic valve prolapse, aortic valve suspension can be performed during the repair of a high ventricular septal defect or sinus of Valsalva aneurysm. In adults with grade I to grade II aortic stenosis or insufficiency, surgery may also be deferred if no clinical symptoms are present. However, if the patient requires mitral valve surgery due to coexisting severe mitral valve disease, concurrent correction of the aortic valve lesion should be considered. Otherwise, after the mitral valve lesion is corrected, the increased left ventricular output into the aorta will exacerbate the hemodynamic changes caused by the aortic valve lesion, leading to left ventricular overload and postoperative left heart failure. Therefore, in rheumatic heart disease patients with combined mitral and aortic valve lesions, the surgical treatment plan should comprehensively consider both valve conditions.
In cases of aortic stenosis and insufficiency, the most dangerous symptoms are angina pectoris and syncope. These symptoms indicate myocardial and cerebral ischemia, and the patient may suddenly experience cardiac arrest or ventricular fibrillation, leading to sudden death. Therefore, patients with a history of angina pectoris or syncope should undergo elective surgery as early as possible.
The aortic valve bears high pressure during closure. Even in cases of simple aortic stenosis, performing commissurotomy often results in significant insufficiency. Additionally, severe aortic stenosis is frequently accompanied by leaflet thickening and calcification, making commissurotomy or valvuloplasty less effective. Therefore, aortic valve lesions often require valve replacement—removing the diseased aortic valve and replacing it with a prosthetic valve. Prosthetic valves in the aortic position have a lower thromboembolism rate than mitral valve replacements due to the冲刷 effect of left ventricular ejection. However, if a mechanical valve is used for aortic valve replacement, lifelong anticoagulation therapy is still required. For bioprosthetic valves, anticoagulation is necessary for at least three months. Whether mechanical or biological, prosthetic valves with larger orifice areas and lower resistance are preferable.
**Surgical Treatment: Aortic Valve Suspension** The prolapsed aortic valve leaflet is often the right coronary or non-coronary leaflet above the defect. After establishing extracorporeal circulation, an oblique transverse incision is made in the anterior wall of the ascending aorta, extending inferiorly to the non-coronary sinus, providing excellent exposure of the aortic valve (Figure 1). A normal sinus is deep, with no abnormalities at the leaflet edges or commissures, whereas a prolapsed leaflet has elongated edges, a shallow sinus, and bulges toward the ventricle. At the commissures, the prolapsed leaflet edge is noticeably thinner (Figure 2).
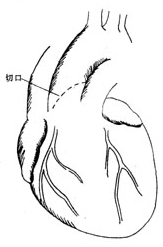
**Figure 1** Schematic diagram of the aortic valve surgical incision
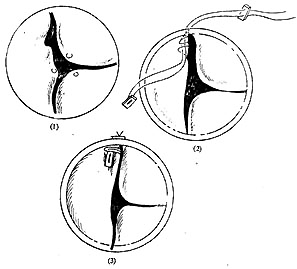
**Figure 2** Aortic valve prolapse leaflet suspension
(1) The prolapsed aortic valve leaflet is redundant, with thin edges at the commissures.
(2) Suturing with pledgeted non-injury needles and threads.
(3) Condition after suspension and fixation of the prolapsed leaflet.
During suspension, use a non-injury forceps to hold one end of the prolapsed leaflet, tighten it toward the commissure, and estimate the degree of prolapse and the range of overlapping sutures for suspension. Then, use a double-ended non-injury needle with polyester or polytetrafluoroethylene pledgets to pass through the overlapping edge of the valve membrane and the leaflet, exiting through another pledget outside the ascending aortic wall, and tie it off. The key points of suspension are: ① The pledget must be placed vertically so that the entire pledget presses against the leaflet to prevent tearing; ② The prolapsed leaflet at the commissure must be about 1mm higher than the adjacent normal leaflet to deepen the sinus and ensure good coaptation of the valve membrane; ③ During suspension, the edge of the prolapsed valve membrane must be slightly tightened, and slight overcorrection can help the valve membrane better withstand the diastolic pressure of the aorta. This suspension method is more precise and effective than using fine silk threads to pull the Morgagni nodules at the center of the edges of the three leaflets to assess the degree of prolapse and the suspension range; ④ If both ends of the leaflet edges show significant degenerative changes, thinning, and loosening, pledget suspension should be performed at both ends of the leaflet.
Aortic valve replacement When the aortic valve is severely damaged and cannot be repaired, aortic valve replacement is required. A disc-shaped mechanical valve or a biological valve can be selected. In cases of degenerative changes or larger annulus, the annulus is often soft and fragile. After removing the diseased valve, 2-0 double-ended non-injury sutures with polyester or polytetrafluoroethylene pledgets should be used, with the needle entering from the aortic side and the pledget placed on the aortic side.
In rheumatic disease, the annular tissue often thickens and becomes very hard, with the annulus shrinking. To implant a larger-diameter prosthetic valve, the prosthetic valve is best placed above the annulus. In such cases, double-ended non-injury sutures without pledgets can be used, with the needle passing from the ventricular side to the aortic side and then threading upward through the sewing ring of the prosthetic valve. After tying the sutures, the prosthetic valve is positioned above the annulus (Figure 3).
After biological valve replacement for the aortic valve, anticoagulation therapy should be administered for 3 to 6 months. For mechanical valve replacement, lifelong anticoagulation therapy is required, maintaining the prothrombin time at 50% of normal.
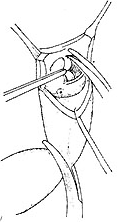
⑴ The aortic valve can be well visualized through an oblique transverse aortic incision, and the aortic valve is excised.
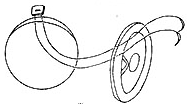
⑵ Double-ended non-injury sutures are used, with the needle entering from the aortic side and the pledget placed on the aortic annulus.

⑶ Double-ended sutures are used, with the needle entering from the ventricular side without pledgets.
Figure 3 Aortic valve replacement






