| disease | Primary Open-angle Glaucoma |
| alias | Chronic Simple Glaucoma, Primary Open Angle Glaucoma |
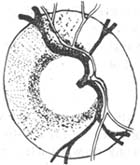
Primary open-angle glaucoma (POAG), also known as chronic simple glaucoma, commonly referred to as "slow single," is a chronic progressive eye disease characterized primarily by elevated intraocular pressure, which subsequently leads to optic nerve damage and visual field defects, ultimately resulting in blindness. In open-angle glaucoma, the anterior chamber angle is mostly wide, with a minority being narrow. Even when intraocular pressure rises, the anterior chamber angle remains open, hence the name.
bubble_chart Epidemiology
Open-angle glaucoma progresses slowly and often lacks obvious symptoms, making early detection difficult. Many patients seek medical attention only when their visual fields have narrowed to a tubular state or when one eye has already gone blind, yet they remain unaware of when the disease began. Some patients are incidentally diagnosed during routine eye examinations, sometimes only after one eye has already lost vision. Therefore, heightened vigilance is necessary for such patients, as the risks are otherwise greater. The condition is slightly more common in men and typically occurs between the ages of 20 and 60, with incidence increasing with age. Relevant data indicate that 24.7% of cases occur in individuals under 40, while 75.3% occur in those over 40. Open-angle glaucoma includes chronic simple glaucoma, low-tension glaucoma, and hypersecretory glaucoma.
Open-angle glaucoma = pathological high intraocular pressure + visual field damage, or = pathological high intraocular pressure + fundus changes.
In chronic simple glaucoma, when intraocular pressure increases, the angle of the anterior chamber does not close and remains open. Although the aqueous humor can fully contact the trabecular surface, it cannot be drained, leading to increased intraocular pressure. The reasons for the increased intraocular pressure may include the following:
1. Changes in the trabecular tissue: sclerosis and degeneration of the trabecular tissue, narrowing of the meshwork, irregularity or even destruction of the trabecular lamellae, enlargement of endothelial cells, degeneration of collagen fibers, degeneration of elastic fibers, and narrowing of the trabecular meshwork spaces.2. Decreased drainage function of Schlemm's canal and its outflow channels or external collector channels.
3. Increased venous pressure: unstable vascular pulse nerves, periodic sympathetic tension, elevated capillary venous pressure, and increased episcleral venous pressure, making aqueous humor drainage difficult.
Chronic simple glaucoma is a group of degenerative eye diseases, manifested as degeneration of the trabecular meshwork, optic disc degeneration, and degeneration of the small pulse supplying the pre- and post-lamina cribrosa. Therefore, even if the intraocular pressure is normal or near normal, visual function can still progressively deteriorate.
Regarding the genetic patterns of open-angle glaucoma:
Modern genetics, particularly pharmacogenetics—studies on the hormone-induced pressure gene and immunogenetics (HLA system)—has two schools of thought on the genetics of open-angle glaucoma. Most believe that open-angle glaucoma is a multifactorial genetic disorder. The occurrence of multifactorial genetic diseases is determined by both genetic and environmental factors. The three measurement parameters of glaucoma—intraocular pressure, C-value, and C/D ratio—are all quantitative traits. Quantitative traits are usually determined by multiple pairs of genes. Therefore, open-angle glaucoma is a multifactorial genetic disorder.
Characteristics of chronic simple glaucoma:
1. No obvious subjective symptoms, with slow progression of the disease;
2. The anterior chamber angle remains open under both high and low intraocular pressure, even if slightly narrow, and its morphology does not change under high pressure;
3. The optic disc primarily shows cup-shaped enlargement;
4. Visual dysfunction mainly refers to visual field injury.
bubble_chart Clinical Manifestations
(1) Subjective Symptoms
In the early stages, there are almost no subjective symptoms. As the condition progresses, symptoms such as visual fatigue, grade I eye distension, and headache may occur. When intraocular pressure fluctuates significantly or reaches a high level, rainbow vision (halos) and blurred vision may also appear. In the advanced stage, the visual field of both eyes narrows, but central vision remains unaffected. The condition is often discovered only when symptoms like night blindness and difficulty moving become apparent. Eventually, vision is completely lost.
(2) Signs
1. Fundus Changes: In the early stages, the fundus may appear normal. As the disease progresses, the physiological cup gradually enlarges and deepens. Blood vessels shift toward the nasal side, and the optic disc becomes pale, with the cup extending to the edge of the disc. Retinal vessels exhibit a "knee-bend" or "climbing" appearance as they cross the disc margin. These three major features are typical manifestations of glaucomatous cupping. It is crucial to identify early changes in the optic disc to facilitate timely diagnosis.
3. Intraocular Pressure: Changes in intraocular pressure manifest as increased fluctuation amplitude and elevated pressure levels. The pressure is usually higher in the early morning, lower in the afternoon, and lowest at midnight, indicating instability. Increased fluctuation amplitude often precedes elevated pressure levels. Pressure changes may occur rapidly, slowly, or remain relatively static at times.
4. Visual Field: Persistent high intraocular pressure directly compresses optic nerve fibers and their blood supply, leading to ischemic atrophy of the optic disc and resulting in visual field defects. The severity of the condition and treatment efficacy can be assessed based on changes in the visual field.
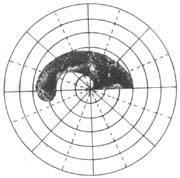
⑴ Central Visual Field Changes: Early detection reveals paracentral scotomas in the Bjerrum area (Figure 3). As the disease progresses, these scotomas expand and curve toward the center, forming arcuate scotomas (Bjerrum scotomas). Eventually, they extend to the nasal central horizontal line, creating a nasal step (Ronne). If such steps appear simultaneously in the upper and lower fields and connect at the nasal center, they form a ring scotoma. This scotoma may gradually widen and merge with peripheral nasal visual field defects.
| | |
| 1. Arcuate Scotoma | 2. Paracentral Scotoma |
Figure 3 Early Visual Field Changes in Glaucoma
| | |
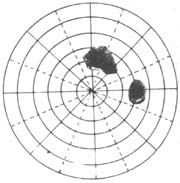
| 1. Nasal Visual Field Defect (Ring Scotoma) | 2. Tubular Visual Field |
Figure 4 Advanced Stage Visual Field Changes in Glaucoma
(2) Changes in peripheral vision: While or shortly after the appearance of scotomas in the central vision, the nasal peripheral visual field narrows (Figure 4), starting from the superior nasal area, then the inferior nasal area, and finally the temporal side. The progression is faster on the nasal side. Sometimes, a quadrant defect or complete defect has already formed on the nasal side, while the temporal visual field still shows no significant changes. If the temporal visual field also progressively narrows, eventually only the central 5–10º remains, resulting in tubular vision (Figure 4). During this period, central visual acuity of 1.0 may occasionally be preserved.
Retinal nerve fiber bundle atrophy: Early-stage band-like atrophy appears beneath the large vascular arches in the superior and inferior temporal regions, visible with an ophthalmoscope as white nerve fiber bundle striations interspersed with widened black striations. In the intermediate stage (second stage) and advanced stage, the white striations completely disappear, leading to quadrant atrophy and diffuse atrophy of the nerve fiber bundles.
Diagnosing glaucoma is a relatively challenging yet crucial task. The types of glaucoma are complex, with varying signs and inconsistent timing of medical visits, which poses certain difficulties for diagnosis, particularly in the early stages, where early detection is especially important.
Diagnosing glaucoma must rely on three key characteristics; otherwise, it may lead to unclear concepts and inaccurate conclusions. The three major characteristics of glaucoma are:
1. Pathological changes in eye function—elevated intraocular pressure.
2. Organic histological changes in the eye—compression of the optic nerve and insufficient blood supply to the optic disc.
3. Visual function impairment—visual field defects in the chronic stage and loss of central vision in the acute stage.
Therefore, it is hoped that diagnosis can be made before irreversible organic changes occur in the optic nerve to facilitate treatment. Additionally, before diagnosis, factors such as a family history of glaucoma, high myopia, hypertension, and diabetes should be considered.
In summary, the diagnosis of glaucoma cannot be confirmed or ruled out based solely on one or two intraocular pressure measurements. A comprehensive understanding of the clinical features and patterns of glaucoma is necessary to make an accurate diagnosis.
Early Diagnosis of Chronic Simple Glaucoma
1. Early diagnosis of glaucoma is extremely important. The so-called early stage refers to when the patient already has the basic conditions and pathological mechanisms for glaucoma. However, subjective and objective symptoms are still not obvious or typical, especially in the early stages of open-angle glaucoma, which often go unnoticed, leading to misdiagnosis or delayed diagnosis and irreversible consequences.
The three major manifestations of open-angle glaucoma are high intraocular pressure, enlarged physiological cup, and visual field defects. The 1985 National Glaucoma Group Jinan Meeting stipulated that if two of these criteria are met, a diagnosis can be made. If only one criterion is present, it should not be considered glaucoma. For example, if intraocular pressure repeatedly falls within the pathological range, it should only be regarded as ocular hypertension.
Early diagnosis of glaucoma should be approached with caution and responsibility. Thorough investigation and comprehensive analysis of various data are required. A diagnosis should not be made hastily based on a single positive result, as this may unnecessarily burden the patient. Conversely, glaucoma should not be ruled out based on one or two negative results, as this may delay treatment. The key points for early diagnosis of chronic simple glaucoma are as follows (Table 1):
Table 1: Early Diagnostic Criteria for Chronic Simple Glaucoma.
| Diagnostic Criteria | Notes | |
| Medical History | Headache, eye distension, rainbow vision, visual fatigue, frequent changes in presbyopia glasses | Consider age, rule out refractive errors, neurasthenia, hypertension |
| Anterior Segment | Peripheral anterior chamber depth > 2/3 CT suggests open-angle glaucoma Peripheral anterior chamber depth < 1/3 CT suggests angle-closure glaucoma | Use narrow light, pay attention to the anterior chamber depth at the 6 o'clock position |
| Fundus | Prominent stirred pulse indicates intraocular pressure above 3.9 kPa (30 mmHg). C/D ≥ 0.6 is pathological, indicating damage to the optic nerve fibers. | Differentiate between stirred pulse and venous pulsation. Pay attention to the vertical (C/DV) and horizontal (C/DH) cup-to-disc ratios. |
| Intraocular Pressure | 1.33~2.8kPa (10~21mmHg) is normal 2.8~2.9kPa (21~29.3mmHg) is suspicious ≥3.12kPa (24mmHg) indicates pathological increase A diurnal difference of 1.04kPa (8mmHg) is pathological | Pay attention to the coefficient of ocular wall rigidity and correct it. Emphasize the difference in intraocular pressure (IOP). A difference of 0.65 kPa (5 mmHg) between both eyes is pathological. For diurnal pressure variations, continuous monitoring for three days is required. |
2. Intraocular Pressure and Tonography: Intraocular pressure is essential for maintaining normal visual function. Normal IOP plays a special role in the optical properties of the eye, intraocular fluid circulation, and lens metabolism. Under normal conditions, aqueous humor production, outflow, and intraocular contents are in a stirred pulse equilibrium. If this balance is disrupted, pathological IOP will occur.
The normal range of IOP is 1.3–2.8 kPa (10–21 mmHg) (measured with a Schiøtz tonometer), but it fluctuates within 24 hours. Generally, IOP is higher in the morning and gradually decreases throughout the day. Sometimes, two peaks may occur within a day, but nighttime IOP is relatively low. The variation should not exceed 0.67 kPa (5 mmHg).
Currently, the Schiøtz tonometer is widely used in China, but attention must be paid to ocular rigidity. Since this tonometer relies on indentation pressure, it may yield falsely high or low IOP readings for eyes with scleral rigidity higher or lower than normal, leading to misdiagnosis. For suspected cases, two weights (5.5 g and 10 g or 7.5 g and 15 g) should be used to measure and calculate the corrected IOP. Highly myopic eyes have a lower scleral rigidity coefficient, so an IOP above 3.33 kPa (25 mmHg) should be considered indicative of glaucoma. The Goldmann applanation tonometer provides more accurate and reliable results compared to the Schiøtz tonometer. Hospitals with the necessary equipment should use applanation tonometers.
Pathological High IOP—The upper limit of normal IOP is 3.12 kPa (24 mmHg). Values exceeding this indicate a 95.45% probability of glaucoma. However, some patients with IOP within the normal range may exhibit significant pathological optic disc cupping and visual field defects. Therefore, determining pathological IOP cannot rely solely on a few measurements but must also consider fundus and visual field findings for a comprehensive diagnosis.
Abnormal IOP: Includes elevated baseline IOP, increased diurnal IOP fluctuations, or both. High IOP should not be diagnosed based on a single measurement. Multiple measurements over time and within a day are necessary. A 24-hour IOP monitoring provides higher diagnostic value.
The following criteria should be applied when measuring IOP:
(1) IOP exceeding 2.8 kPa (21 mmHg) (Goldmann applanation tonometer) or 2.99 kPa (23 mmHg) in supine position, with specific fundus changes (including retinal nerve fiber layer defects and optic disc changes) or glaucoma-specific visual field defects, can confirm a diagnosis of primary open-angle glaucoma.
(2) If peak IOP does not exceed the upper normal limit but the above fundus and visual field changes are present, and other diseases causing such changes are excluded, a diagnosis of low-tension glaucoma can be made. However, such patients may also have positional hypotension, hemodynamic crises, thin corneas, large vitreous cavities, or myopia.
Tonography for Glaucoma Diagnosis: Tonography is used to demonstrate the mechanism of elevated IOP in chronic simple glaucoma. Impaired aqueous outflow is the key factor causing IOP elevation. Therefore, when IOP rises, the aqueous outflow coefficient decreases, aiding in the diagnosis of chronic simple glaucoma.
3. Provocative Tests: For suspected glaucoma cases with normal IOP, provocative tests can be used to induce IOP elevation for confirmation. Glaucoma provocative tests target the disease mechanisms of different glaucoma types. For suspected cases, corresponding methods are applied to induce IOP elevation, facilitating early diagnosis.
⑴ Provocative test for suspected angle-closure glaucoma
1) Dark Room Test: Dim light is a harmful stimulus for glaucoma. The dark room test is relatively safe, requires no special equipment, and is simple to perform. The mechanism of increased intraocular pressure may involve pupil dilation, where the thickened iris root blocks the angle, and increased pupillary block due to iris-lens contact. In chronic angle-closure glaucoma, iris bombe remains the primary cause of elevated intraocular pressure due to pupillary block. Plateau iris syndrome may be related to mechanical obstruction of the angle by the iris during dilation.
Method: The subject wears an eye patch and sits quietly in a dark room for 1–2 hours (1 hour for younger individuals, 2 hours for older individuals with smaller, less dilatable pupils). Sleeping is prohibited to maintain alertness, as it may affect test results. After the test, intraocular pressure should be measured promptly under red light (dim illumination). An increase of 1.25 kPa (8 mmHg) is considered positive. For positive cases, both the difference in intraocular pressure before and after the test and changes in the angle should be observed. A narrow slit beam is recommended for optimal observation. If intraocular pressure rises, 1% pilocarpine should be administered to lower it. Elderly patients with cardiovascular conditions should avoid this test.
2) Reading Test: First, measure intraocular pressure, then instruct the patient to read small-font text (No. 5 font) at the closest possible distance for 1 hour (presbyopic patients may wear glasses). A post-test increase of 1.33–2.0 kPa (10–15 mmHg) is considered positive.
The mechanism of intraocular pressure elevation during the reading test involves ciliary body rotation around the iris during accommodation, causing the iris root to press against the trabecular meshwork.
3) Prone Test: The patient lies face down on a bed (Figure 1) with the forehead resting on the back of the hand or a pillow, keeping eyes closed while awake for 1 hour. An increase of 1.06 kPa (8 mmHg) after 1 hour is considered positive. The mechanism may involve anterior displacement of the lens, pressing against the iris and exacerbating pupillary block.
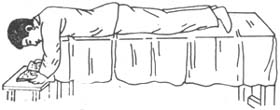
Figure 1: Prone Test
4) Head-Down Prone Dim-Light Reading Test: A simple, safe, and effective test.
Method: Measure intraocular pressure first, then have the patient lie prone on a bed tilted at a 5-degree angle and read for 1 hour. The chin should rest slightly on the lower edge of the bed, and the occiput should be level with or slightly below the shoulder plane.
Lighting should be dim, using backlighting or drawn curtains.
The reading distance should allow clear visibility of small text.
After 1 hour, measure intraocular pressure again in a supine position. An increase of 1.46 kPa (11 mmHg) or more is considered pathological.
The effect of posture on intraocular pressure may be secondary to increased intraocular blood pressure. Reading induces accommodation, causing the ciliary body to rotate along the iris ridge and pull the iris root against the trabecular meshwork. The prone position combined with reading may also displace the lens forward, increasing lens-iris contact and pupillary block.
5) Mydriasis Test: This is one of the diagnostic methods for early glaucoma but should not be used routinely due to its risks. It may trigger acute angle-closure glaucoma or even irreversible angle closure. Therefore, it should be performed cautiously without adequate emergency preparedness.
Method: Instill one drop of 2% homatropine, and begin measuring intraocular pressure when the pupil dilates to 5 mm. Measure every 15 minutes initially, then every 2 hours for a total of three measurements. The following points should be noted during the test: ① Measure and record pupil size simultaneously with intraocular pressure; ② Do not perform mydriasis tests on both eyes simultaneously; ③ If intraocular pressure rises above 3.99 kPa (30 mmHg) after dilation, examine the anterior chamber angle under high-pressure conditions; ④ After the test, promptly induce miosis, and administer 250 mg diamox orally if necessary; ⑤ Only allow the patient to leave after the pupil constricts and intraocular pressure returns to normal, otherwise there is a risk of elevated pressure; ⑥ Even if the test is negative, acute episodes may still occur within 1–2 days.
(2) Provocative tests for suspected open-angle glaucoma
1) Water-drinking test: The method is simple, requires no special equipment, and causes no harm to the body. Its disadvantage is the low positive rate and limited diagnostic value.
Fasting for 8 hours before the test,Jinfu medication. Drink an appropriate amount of warm water (calculated as 14ml per kilogram of body weight) within 5 minutes. After drinking, measure intraocular pressure every 15 minutes for a total of four times. Tonography begins 20 minutes after drinking. An intraocular pressure increase of 0.8 kPa (6 mmHg) is considered a suspicious pathological state, while an increase of 1.06 kPa (8 mmHg) is a significant pathological state. A pressure-to-outflow ratio ≥120 is regarded as pathological.
The mechanism of intraocular pressure elevation during the test is due to blood dilution causing a decrease in osmotic pressure, leading to increased aqueous humor inflow into the eye.
2) Intravenous glucose injection test: The mechanism of intraocular pressure elevation is the same as the water-drinking test.
Method: Administer 14ml of 50% glucose solution per kilogram of body weight intravenously over 3–5 minutes. Measure intraocular pressure at 15, 25, 35, and 50 minutes afterward. The diagnostic criteria are the same as above.
3) Tolazoline test: After measuring intraocular pressure, inject 1ml (10mg) of tolazoline subconjunctivally, then measure intraocular pressure every 10 minutes for 60 minutes. A post-injection intraocular pressure increase of ≥0.8 kPa (6 mmHg) is considered positive. The mechanism of pressure elevation is vasodilation, which increases aqueous humor production and raises intraocular pressure.
Although the above provocative tests for suspected open-angle glaucoma are still used clinically, their low positive rate and potential for false positives mean they can only serve as clinical references.
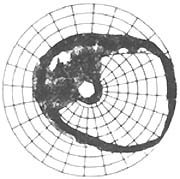
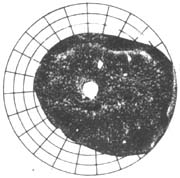
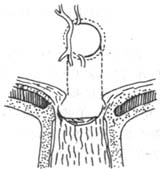
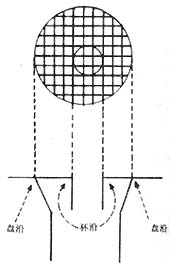
4. Glaucomatous optic disc changes (Figure 2): Glaucomatous cupping and atrophy of the optic disc are reliable diagnostic criteria. Common morphological changes include: ① Enlarged optic disc cupping: Vertical oval shape, eccentric cup position, progressive deepening and widening of the cup, and striated appearance of the lamina cribrosa are strongly associated with visual field defects and large cups; ② Cup-to-disc ratio should be expressed numerically (e.g., 0.3, 0.4, 0.5...) and recorded in both vertical and horizontal directions. The width of the cup itself and the disc rim should be noted (e.g., vertical C/DV = superior disc rim, vertical cup diameter, inferior disc rim; horizontal C/DH = temporal disc rim, horizontal cup diameter, nasal disc rim). A 5-grid division method should be used to illustrate cup size, shape, and position (Figure 2); ③ Steep cup margins are drawn with solid lines, while sloping margins use dashed lines. Cup size is measured by contour, not color—the point where small vessels bend downward from the disc surface marks the cup's edge. Beyond the cup-to-disc ratio, special attention should be paid to rim width, notching, saucerization, disc hemorrhages, and retinal nerve fiber layer defects; ④ Gradually adopt slit-lamp biomicroscopy, photography, and stereoscopic color photography to observe and document fundus changes; ⑤ Mismatch between central pallor extent and pallor/cup ratio in the optic disc; ⑥ Optic disc hemorrhage; ⑦ Peripapillary atrophy; ⑧ Pulsation of the central retinal artery.
|
|
|
|
| 1. Changes in the cup-to-disc ratio of glaucoma | 2. Glaucomatous cup | 3. Measurement of the glaucomatous cup-to-disc ratio |
Figure 2: Glaucomatous optic disc changes
The vertical diameter of the optic disc excavation is greater than the horizontal diameter: Normally, few people have a vertical oval shape; most have a horizontal oval. A vertical oval shape is more commonly seen in glaucoma, emphasizing the importance of the vertical cup-to-disc ratio. The expansion of the vertical excavation indicates damage to the optic nerve tissue in the root of the nose area of the optic disc, causing narrowing of the edges in this region.
Enlargement and deepening of the optic disc excavation: Physiological excavation does not progress. If it progresses, it is pathological. The pattern of progression is as follows: Before glaucoma develops, the lamina cribrosa is not visible. In the early stages of the disease, the excavation deepens centrally, exposing the lamina cribrosa, and then no longer deepens but expands at the base, exposing more of the lamina cribrosa.
Atrophy of the optic disc rim: Early damage occurs in the nerve fiber bundles at the superior and inferior edges of the optic disc. The expansion of the excavation merely reflects the underlying issue of tissue atrophy and loss. Therefore, for suspected glaucoma cases, focus should be placed on examining the superior and inferior rims of the disc, especially the temporal superior and temporal inferior disc rims. Even if the excavation is not large, thinning of the disc rim tissue, notches, uneven width, or tissue loss all indicate the presence of glaucoma.
Peripapillary atrophy: Caused by insufficient blood supply from the short posterior ciliary stirred pulse.
Stirred pulse pulsation: The appearance of pulsation indicates a pathological state, occurring only when intraocular pressure is high or stirred pulse pressure drops to a certain level.
5. Observation of glaucomatous retinal nerve fiber layer defects (RNFLDs): The destruction of the optic nerve fiber layer caused by glaucoma can occur before optic disc excavation and visual field defects. Therefore, observing RNFLDs can serve as an important sign for early diagnosis of open-angle glaucoma. As the glaucoma disease progresses, NFL atrophy also develops. Hence, it can predict disease progression and the effectiveness of treatment, and it is closely related to the morphology of the optic disc and visual field defects. For examination methods, an ophthalmoscope can be used, while a fundus camera provides a wider observation range with clear focus. Using red-free light can enhance visibility. Additionally, fundus images can be recorded for comparison or group discussions, making it an ideal examination method.
Manifestations of pathological optic nerve fiber bundle defects:
⑴ Localized RNFLD atrophy: Faint fissures in the superior and inferior arcuate fiber bundles, often multiple and resembling "comb-like" patterns.
⑵ Diffuse RNFLD atrophy: Diffuse thinning of the retinal nerve fiber layer, with a darker color.
6. Fluorescein angiography of the glaucomatous optic disc: In confirmed cases of open-angle glaucoma, the positive rate of fluorescein angiography is 75%. No abnormalities are found in patients excluded from glaucoma, so it has little significance for early diagnosis.
7. Visual field defects: Visual field changes are crucial for the diagnosis, treatment, and prognosis of glaucoma. The visual damage in glaucoma results from an imbalance between intraocular pressure and intravascular pressure in the nerves, leading to optic nerve ischemia. Glaucomatous visual field defects are caused by optic nerve damage due to impaired circulation in the optic disc.
The significance of visual fields in glaucoma diagnosis: Primarily for chronic simple glaucoma. Carefully measured visual field tests and some specialized visual field tests can detect early visual field changes. Various forms of visual field defects represent different stages of glaucoma, and understanding their characteristics has clinical significance.
In the early stages of chronic simple glaucoma, visual field defects manifest as small relative or absolute scattered paracentral scotomas. As the condition progresses and intraocular pressure continues to rise, localized scotomas appear along the arcuate nerve fiber bundles, most of which are not connected to the physiological blind spot. Subsequently, these scotomas increase in number, expand, and merge to form arcuate scotomas or nasal step defects. With further progression of the disease, the upper and lower arcuate scotomas connect, forming a ring scotoma. The advancement of the nasal step signifies the transition of chronic simple glaucoma to the advanced stage, eventually leading to central island vision or residual small temporal islands. By the time only a central island remains, central vision may be lost, leaving only a very small temporal island.
Any visual field defect with a sloping edge indicates active disease. If the edge of the visual field defect is steep, it suggests slow progression of the lesion.
8. Gonioscopy: The width, openness, closure, shortening, and extent of peripheral anterior synechiae of the entire angle circumference should be described and illustrated in clock-hour positions, noting whether the image is erect or inverted. The morphology of the peripheral edge (convex or concave) should also be recorded, using the Scheie classification for pigment grading. First, observe under static conditions to assess the angle width without altering its natural state. Then, perform dynamic observation (e.g., indentation gonioscopy) to determine the degree and extent of angle opening, closure, and peripheral anterior synechiae. Record the intraocular pressure and medication status during the examination.
bubble_chart Treatment Measures
1. Drug Therapy: Particularly in the early stages, the precision of visual field examinations is crucial. For those with progressive visual field defects, prompt and appropriate drug therapy should be administered. By using the 24-hour diurnal intraocular pressure curve, medications can be added before the peak to avoid concealed visual function damage.
The drug treatment for chronic simple glaucoma primarily involves topical medications, starting with low concentrations. If therapeutic goals are not achieved, the concentration can be gradually increased or additional medications can be added.
If intraocular pressure remains uncontrolled with medication or if there is progression of optic disc or visual field damage, surgical treatment may be considered. Options include trabeculectomy or other filtering surgeries, and laser therapy may also be an option.
Principles of Treatment for Chronic Simple Glaucoma
Early Stage: If intraocular pressure is controlled below 2.66 kPa (20 mmHg), the visual function of most cases can avoid further damage. Treatment mainly relies on medication, and surgery is not necessary.
Intermediate Stage [Second Stage]: Intraocular pressure should be controlled around 2.13 kPa (16 mmHg). If drug therapy is inadequate and visual function suffers progressive damage, surgical treatment is required.
Advanced Stage: If intraocular pressure remains consistently above 2.13 kPa (16 mmHg), blindness is often unavoidable. Even with lower intraocular pressure, visual function may continue to decline, often requiring both medication and surgical treatment.
Common Medications for Chronic Simple Glaucoma
(1) β-Adrenergic Receptor Blockers: Ophthalmic use typically involves 0.25%–0.5% timolol, administered 1–2 times daily. This can reduce general increases in intraocular pressure and bring it back to normal. For cases with extremely high intraocular pressure, combination therapy with other pressure-lowering medications is necessary.
(2) L-Epinephrine: A 1–2% solution administered 1–2 times daily can maintain a pressure-lowering effect for 12–24 hours. Its advantage is that it does not cause pupil constriction or ciliary muscle spasm, thereby reducing many side effects.
(3) Pilocarpine: Commonly used at 1–2%, or 4% solution or ointment if necessary, administered 4–6 times daily. Frequent administration or high concentrations should be avoided to minimize the risk of ciliary muscle spasm.
(4) Carbonic Anhydrase Inhibitors: These reduce aqueous humor production. Commonly used drugs include acetazolamide (Diamox) tablets, often used short-term before surgery. To avoid systemic effects, long-term use is generally not recommended.
Timolol is a potent beta-blocker, with an effect more than 8 times stronger than propranolol. It is currently the best beta-blocker for lowering intraocular pressure. Its mechanism involves inhibiting aqueous humor production, and a single topical dose can maintain a pressure-lowering effect for at least 24 hours.
2. Surgical Treatment: If intraocular pressure cannot be controlled with medication, if the patient cannot tolerate the medication, or if visual function continues to deteriorate despite drug therapy, surgical treatment should be considered. Surgical options include trabeculectomy, lamellar scleral resection, or other filtering surgeries. Currently, trabeculectomy is widely used.
(1) Differential Diagnosis of Open-Angle and Angle-Closure Glaucoma
After confirming primary glaucoma, it is essential to determine its type and establish the correct management plan. Generally, acute angle-closure glaucoma is not misdiagnosed as open-angle glaucoma.
The key points for differentiation:
1. Medical history: Comprehensive analysis based on the condition and characteristics of the episodes.
2. General conditions
Age: Primary glaucoma in individuals under 30 was previously termed open-angle glaucoma but is now referred to as congenital glaucoma. Those around 50 or older are more likely to have angle-closure glaucoma.
Gender: Angle-closure glaucoma is more common in females, while open-angle glaucoma is more common in males. For females over 40, angle-closure should be considered; males under 30 likely have congenital glaucoma.
Refractive status: Hyperopia is more associated with angle-closure glaucoma, while myopia is more linked to open-angle glaucoma. High hyperopia increases the likelihood of angle-closure glaucoma, whereas high myopia is more prone to open-angle glaucoma.
3. Anterior segment manifestations: A narrow cornea (≤10.5mm) and shallow anterior chamber (<2.5mm) with iris bombe suggest angle-closure glaucoma. A normal anterior chamber and flat iris indicate open-angle glaucoma.
4. Anterior chamber angle: Open-angle glaucoma features a wide angle without adhesions, remaining open even with elevated intraocular pressure (IOP). Angle-closure glaucoma has a narrow angle that closes when IOP rises and reopens when IOP drops, revealing the trabecular meshwork.
In chronic angle-closure glaucoma, most or all of the angle is adherent.
5. Tonography: In open-angle glaucoma, the aqueous outflow coefficient is minimally affected by IOP fluctuations. In angle-closure glaucoma, when the angle is closed and IOP is high, the outflow coefficient (C-value) is low; when the angle opens and IOP drops, the C-value increases.
6. IOP and fundus: Very high IOP (e.g., 7.98 kPa or 60 mmHg) with a normal optic disc suggests angle-closure glaucoma. Open-angle glaucoma typically reaches high IOP only in advanced stages, often accompanied by enlarged optic disc cupping. Alternatively, even with moderate IOP (~3.99 kPa or 30 mmHg), significant cupping may indicate open-angle glaucoma.
Below is a comparative table (Table 2) for differentiating chronic angle-closure glaucoma and chronic open-angle (simple) glaucoma:
Table 2: Differential Diagnosis of Chronic Angle-Closure Glaucoma and Chronic Simple Glaucoma
| Chronic Angle-Closure (Iris Bombe Type) | Chronic Angle-Closure (Plateau Iris Type) | Chronic Simple | |
| Medical History | Recurrent episodes | Few symptoms in advanced stages | Mostly asymptomatic |
| Disease Course | Often episodic high IOP | Slow IOP elevation | Progressive IOP elevation |
| IOP | Normal or elevated in early stages | Same as left | Slightly elevated in early stages |
| Anterior Chamber | Shallow | Normal centrally, shallow peripherally | Mostly deep |
| Anterior Chamber Angle | Narrow angle with adhesions; angle changes with IOP fluctuations | Short angle with creeping adhesions; angle changes with IOP fluctuations | Mostly wide angle, rarely narrow; angle remains unchanged with IOP fluctuations |
| Fundus | Generally normal | Early stage normal | Early stage C/D ≥ 0.6 |
| Visual field | Generally normal | Same as above | Early glaucomatous changes |
(II) Differential diagnosis between chronic angle-closure glaucoma and the chronic stage of acute angle-closure glaucoma
Chronic angle-closure glaucoma
1. In the early stage, intraocular pressure elevation is fluctuating and can resolve spontaneously.
2. Even under high intraocular pressure, the angle is not completely closed, and a considerable range of the ciliary body band can still be seen.
3. Pupil is grade I dilated, with no obvious iris atrophy.
Chronic stage of acute angle-closure glaucoma
1. It results from untreated or inadequately treated acute angle-closure glaucoma, with a history of acute attack.
2. Intraocular pressure may remain at 3.9–5.32 kPa (30–40 mmHg) and does not resolve spontaneously.
3. There may be varying degrees of angle adhesion.
4. Most cases show residual segmental iris atrophy, glaucomatous flecks, and vertically dilated pupils as acute signs.
(III) Differential diagnosis between glaucomatocyclitic crisis and acute angle-closure glaucoma
Glaucomatocyclitic crisis
1. This condition is a type of secondary open-angle glaucoma, mostly occurring unilaterally in middle-aged patients, with recurrent episodes in the same eye, though bilateral cases also exist.
2. Episodic intraocular pressure elevation, with each episode lasting about 1–14 days, resolving spontaneously. Symptoms are generally mild, with only blurred vision or halos.
3. Intraocular pressure usually rises to 5.32–7.8 kPa (40–60 mmHg), but can reach as high as 10.64 kPa (80 mmHg). The elevation does not correlate proportionally with symptoms or vision. Despite high pressure, ocular discomfort is grade I, without nausea, vomiting, severe headache, or eye pain.
4. Each episode presents with grade I ciliary congestion and a few small or medium-sized round gray-white keratic precipitates. The angle remains open during pressure elevation, with reduced C-value; C-value normalizes when pressure normalizes.
5. The affected pupil is dilated during episodes, but no posterior synechiae form despite recurrent attacks.
6. Visual field: Generally normal; fundus also normal. Any changes may indicate coexisting chronic simple glaucoma.
7. During remission, all provocative tests are negative.
(IV) Differential diagnosis between acute stage of angle-closure glaucoma attack and glaucoma secondary to iridocyclitis
The acute stage of angle-closure glaucoma often presents with some signs of iritis, while acute iritis may sometimes be accompanied by mild intraocular pressure elevation. These two conditions differ fundamentally in treatment, and misdiagnosis can lead to severe consequences.
Key differences:
1. In glaucoma, intraocular pressure is extremely high, and the eye is rock-hard; in iridocyclitis, pressure is normal or mildly elevated.
2. In glaucoma, the pupil is dilated and irregular; in iridocyclitis, it is smaller.
3. In glaucoma, keratic precipitates are pigmented granules; in iridocyclitis, they are inflammatory exudates, appearing gray-white.
4. After an acute glaucoma attack, the classic triad of glaucoma signs is often present, which is absent in iridocyclitis.
5. Treatment differs fundamentally: acute angle-closure glaucoma requires miotics to open the angle and rapidly lower pressure, while acute iritis requires prompt mydriatics to prevent posterior synechiae.