| disease | Mixed Acid-base Imbalance |
| alias | Mixed Acid-base Balance Disorder, Mixed Acid-base Balance Disorder |
Mixed acid-base disturbances refer to the simultaneous presence of two or more simple acid-base imbalances. In mixed acid-base disorders, the original compensatory responses no longer exist, and the pathophysiological changes are more complex, potentially leading to atypical clinical manifestations. Therefore, a preliminary diagnosis should be made by carefully reviewing the patient's medical history and analyzing blood gas results. Mixed acid-base disturbances can involve various combinations, but it is clearly impossible for respiratory acidosis and respiratory alkalosis to occur simultaneously. When two primary disorders shift the pH in the same direction, the deviation from normal becomes more pronounced. For example, a patient with metabolic acidosis combined with respiratory acidosis will have a lower pH than with either disorder alone. When two disorders shift the pH in opposing directions, the plasma pH depends on the dominant disorder, and the magnitude of the change is less pronounced than with a single disorder due to the counteracting effect of the other. If the pH shifts caused by the two disorders exactly cancel each other out, the patient's plasma pH may remain normal, as seen in metabolic acidosis combined with respiratory alkalosis.
bubble_chart Clinical Manifestations
Mixed acid-base disorders commonly include the following five types.
(1) Respiratory acidosis combined with metabolic acidosis
Respiratory acidosis combined with metabolic acidosis is seen in: ① Chronic respiratory acidosis such as obstructive pulmonary disease accompanied by toxic shock with lactic acidosis; ② Cardiac and respiratory arrest leading to acute respiratory acidosis and lactic acidosis due to hypoxia. This mixed acid-base disorder can cause a significant drop in plasma pH, a decrease in plasma [HCO3
-], and an increase in Pco2. For example, a patient's plasma pH is 7.0, Pco2 is 11.3 kPa (85 mmHg), [HCO3-] is 14.4 mmol (mEq)/L, and B.E. is -12 mmol (mEq)/L.(2) Respiratory acidosis combined with metabolic alkalosis
Respiratory acidosis combined with metabolic alkalosis is seen in patients with chronic obstructive pulmonary disease who develop hypercapnia and, due to pulmonary heart disease and heart failure, use diuretics such as furosemide or ethacrynic acid, leading to metabolic alkalosis. This is also a common scenario encountered in respiratory, cardiac, and renal departments. The patient's plasma pH may be normal, slightly elevated, or slightly decreased, but both [HCO3-] and Pco2 are significantly elevated. The increase in [HCO3-] is characteristic of metabolic alkalosis, while the increase in Pco2 is characteristic of respiratory acidosis, but their ratio may remain unchanged or vary slightly. For example, a patient's plasma pH is 7.4, Pco2 is 60 mmHg, plasma [HCO3-] is 34 mEq/L, and B.E. is +14 mEq/L.
(3) Respiratory alkalosis combined with metabolic acidosis
This mixed acid-base disorder can be seen in: ① Patients with renal insufficiency who have metabolic acidosis and, due to fever, hyperventilate, leading to respiratory alkalosis, such as acute renal failure caused by Gram-negative bacillus sepsis accompanied by high fever. ② Patients with liver insufficiency may hyperventilate due to stimulation by NH3, while also developing lactic acidosis due to metabolic disorders. ③ Excessive doses of salicylic acid causing metabolic acidosis while stimulating the respiratory center, leading to hyperventilation. The plasma pH may be normal, slightly elevated, or slightly decreased, but both plasma [HCO3
(4) Respiratory alkalosis combined with metabolic alkalosis
This mixed acid-base disorder can be seen in: ① Patients with fever and vomiting, who have respiratory alkalosis caused by hyperventilation and metabolic alkalosis caused by vomiting. ② Patients with cirrhosis and ascites, who hyperventilate due to the stimulation of NH3, and when diuretics are used or vomiting occurs. In this type, the plasma pH value is significantly elevated, plasma [HCO3-] may increase, and Pco2 may decrease. The increase in [HCO3-] is characteristic of metabolic alkalosis, while the decrease in Pco2 is characteristic of respiratory alkalosis. For example, a patient's plasma pH is 7.68, Pco2 is 29 mmHg, plasma [HCO3-] is 38 mEq/L, and B.E is +14 mEq/L.
(5) Metabolic acidosis combined with metabolic alkalosis
Respiratory acid-base disorders cannot coexist, but metabolic acid-base disorders can occur simultaneously. For example, in patients with acute renal failure who experience vomiting or undergo gastric suction, both metabolic acidosis and metabolic alkalosis may be present. However, the plasma pH, [HCO3-], and Pco2 may all be within the normal range or slightly higher or lower.
Mixed acid-base balance disorders are relatively complex and can only be diagnosed after thorough research and analysis of the disease's progression. Nevertheless, a few mixed acid-base balance disorders remain difficult to identify. Currently, even in well-equipped hospitals in China, approximately 2.2% of cases still cannot be definitively diagnosed clinically. This indicates that further research is needed in the principles and techniques of diagnosing acid-base balance disorders.
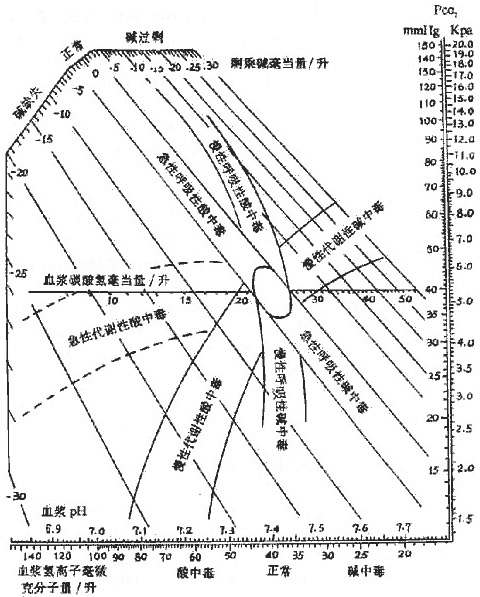
Figure 1: Schematic diagram of changes in plasma pH, Pco2, and HCO3- in various types of acid-base balance disorders.





