| disease | Primary Heart Tumor |
Primary benign heart tumors account for about 70% of primary heart tumors, and most cases can be cured by surgery. Among benign heart tumors, more than half are cardiac myxomas, while other benign heart tumors include lipomas, hemangiomas, fibromas, hamartomas, and teratomas. Myxomas are most commonly found in the left atrial cavity, accounting for about 75% of all cardiac myxomas, followed by right atrial myxomas, which account for about 20%. Ventricular myxomas and multiple cardiac myxomas are very rare.
bubble_chart Epidemiology
The incidence of this disease has not been accurately documented. According to Western literature, approximately 1 case of cardiac myxoma is found in every 350 cases of rheumatic heart disease with mitral valve lesions. However, domestic literature reports a much higher incidence rate compared to Western countries. This disease is more common in women, with a male-to-female ratio of about 1:1.5 to 2.0, and the most common age of onset is between 30 and 50 years.
bubble_chart Pathological Changes
Cardiac myxoma is a primary benign tumor, mostly originating from primitive endothelial cells or endocardial membrane cells near the fossa ovalis of the interatrial septum. The tumor has a stalk of varying width, connecting to the fossa ovalis of the interatrial septum (Figure 3). A few myxomas may originate from other parts of the cardiac chamber. Although the pathological characteristics of myxoma are benign, its biological behavior has a low-grade malignant tendency, mainly manifested by rapid growth and invasive growth characteristics. Incomplete surgical resection often leads to the possibility of local recurrence and malignant transformation.
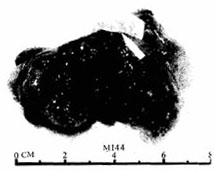
Figure 3 Gross examination of myxoma
The arrow points to the stalk attached to the interatrial septum tissue
Myxoma can be divided into three types by gross examination: ① Mass type: The tumor is a solid mass with a complete membrane, and occasionally tumor fragments may detach and cause systemic embolism. ② Polyp type: The tumor is polyp-like and grape-like, covered by an endothelial cell layer. This tumor is fragile and may detach, causing systemic and pulmonary embolism. ③ Mixed type: A combination of the above two types.
Under microscopy, the tumor surface is covered by endothelial cells, and the tumor body mainly consists of polysaccharides and myxoid matrix, containing plasma cells, lymphocytes, and polygonal cells, with fibroblasts and phagocytic cells scattered in a reticular pattern in loose connective tissue. The tumor body also has microvessels extending into it and hemosiderin deposition (Figure 4). If it is a myxoid fleshy tumor, the tumor cells are abundant, with varied cell morphology, large and deeply stained nuclei, and nuclear division phases. Tumor cells may invade small blood vessels to form tumor emboli.
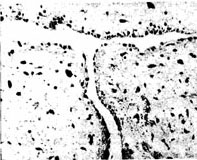
Figure 4 Microscopic examination shows plasma cells within the myxoid matrix
Lymphocytes and polygonal cells, with capillaries
Pathophysiological changes: Mainly determined by the location, size of the tumor, and the degree of obstruction of the atrioventricular valve orifice. Tumor obstruction of the mitral valve orifice can lead to blood flow obstruction through the mitral valve, resulting in clinical manifestations similar to mitral stenosis, such as pulmonary congestion and hypertrophy of the left atrium and right ventricle. If the tumor obstructs the tricuspid valve orifice, the clinical manifestations are similar to tricuspid stenosis, tricuspid valve prolapse, constrictive pericarditis, and myocarditis symptoms. Additionally, the detachment of myxoma fragments can cause systemic and pulmonary embolism.
bubble_chart Clinical ManifestationsThe main manifestations include palpitation, shortness of breath, and chest tightness after exertion, similar to the symptoms of mitral stenosis. The disease progresses relatively quickly, eventually leading to heart failure. Dizziness or transient syncope is caused by the tumor obstructing the mitral valve orifice, leading to transient cerebral hypoperfusion. Rest or changes in body position can alleviate these symptoms. Acute obstruction of the mitral valve orifice by the tumor can cause a sudden increase in left atrial and pulmonary venous pressure, resulting in acute left heart failure, pulmonary edema, and hemoptysis. Long-term elevation of left atrial pressure, accompanied by increased pulmonary artery pressure and right heart failure, presents as jugular vein distension, lower limb edema, hepatosplenomegaly, and even ascites. Tumor emboli entering the systemic circulation can cause embolisms in the brain, kidneys, mesentery, and lower limb vessels. Additionally, about half of the patients may experience low-grade fever, grade I anemia, weight loss, and poor appetite, which may be related to hemorrhage, degeneration, and necrosis within the tumor or the body's allergic reaction to the myxoma.
Signs: Most patients do not exhibit a mitral facies, and blood pressure is normal. Patients with right heart failure may show jugular vein distension and lower limb edema, and in severe cases, hepatosplenomegaly or ascites may be palpable.
Cardiac auscultation: The heart rhythm is regular, and diastolic, systolic, or biphasic murmurs can be heard at the apex. In some patients, the nature and intensity of the heart murmur may change with body position, a feature often emphasized by most scholars as a diagnostic criterion for myxoma. The diastolic murmur at the apex is relatively short, localized, and does not widely propagate, with an accentuated first heart sound. In some cases, a tumor plop may be heard. When pulmonary hypertension is present, an ejection sound can be heard at the pulmonary valve area, with an accentuated or split second heart sound. Right atrial myxoma may present with a diastolic murmur at the tricuspid area.
bubble_chart Diagnosis(1) Phonocardiogram In some patients, the intensity of the murmur may change with different body positions.
(2) Electrocardiogram The electrocardiogram shows no characteristic manifestations and may be normal or show signs of left atrial and right ventricular hypertrophy and myocardial damage. Atrial fibrillation is rare.
(3) Cardiac X-ray Findings The findings are similar to those of mitral valve disease, with pulmonary congestion and heart size increasing from grade I to grade II, mainly manifested as left atrial and right ventricular enlargement. Barium swallow examination of the esophagus may show grade I to grade II esophageal indentation.
(4) Echocardiogram Echocardiography is a non-invasive examination with specific diagnostic value for cardiac myxoma. Its main manifestations are: ① Enlargement of the left atrial cavity. ② Dense cloud-like abnormal echoes appear in the heart cavity. ③ These abnormal echoes change with the opening and closing of the atrioventricular valves. During diastole, the abnormal echoes of the tumor may protrude into the atrioventricular valve orifice or partially into the left or right ventricle. During systole, the tumor re-enters the atrial cavity (Figures 1 and 2).
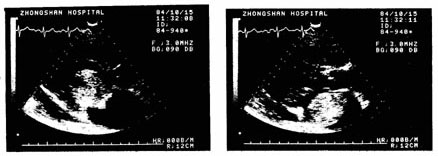
Figure 1 Sector Echocardiogram

Figure 2 M-mode Echocardiogram showing dense cloud-like abnormal echoes in the left atrial cavity
(5) Cardiac Angiography Intracardiac angiography can provide reference information on the location, shape, size, and range of motion of intracardiac myxomas. However, due to the dilution of the contrast agent in the heart cavity and the overlapping shadows of the heart chambers, the imaging may be suboptimal. Additionally, there are certain difficulties in distinguishing it from left atrial thrombus, and the examination equipment is complex and costly. Therefore, the diagnosis of intracardiac myxoma has been replaced by echocardiography.
bubble_chart Treatment Measures
Although cardiac myxomas are benign, incomplete resection can lead to postoperative recurrence. Careless handling during surgery, causing tumor fragments to detach, can result in systemic embolism postoperatively. Currently, the surgical treatment for cardiac myxomas advocates for the complete removal of the tumor and the atrial septum tissue at the base of the tumor pedicle under direct vision during extracorporeal circulation. To avoid the potential detachment of tumor fragments during surgery, after the tumor is removed, all chambers of the heart should be thoroughly rinsed with a large amount of saline, and careful inspection should be conducted to check for the presence of multiple myxomas and to examine whether there is any enlargement of the atrioventricular valves and annuli. In some cases, myxomas may cause valve membrane injury or myxoid degeneration of the valve membrane, sometimes necessitating additional valve membrane plasty, annuloplasty, or artificial valve replacement to prevent postoperative valve insufficiency.
The choice of atrial incision can be made between a right atrial incision, a left atrial incision through the interatrial groove, or a biatrial incision. When the tumor is large, a biatrial incision is preferable.
【Postoperative Complications and Their Management】 Apart from the general complications that may arise from extracorporeal circulation surgery, the most common complications following cardiac myxoma surgery are: ① Arrhythmias and atrioventricular block: These are generally transient, with ventricular premature beats treated with intravenous lidocaine. Transient complete atrioventricular block is managed with intravenous isoproterenol to maintain heart rate, and if necessary, a temporary transvenous endocardial pacemaker is placed until sinus rhythm is restored, after which the pacing leads are removed. ② Systemic embolism: Often caused by the detachment of tumor fragments, embolism in the major cerebral vessels can lead to cerebral hypoxia, edema, and necrosis, resulting in unconsciousness or even death. Embolism in the vessels of other vital organs, if unresponsive to vasodilators and anticoagulant therapy, may require surgical removal of the embolus.
Primary malignant heart tumors are mainly sarcomas, including myxosarcoma, rhabdomyosarcoma, lymphosarcoma, and malignant hemangioma, among which rhabdomyosarcoma is the most common. These tumors tend to occur at a younger age and are slightly more common in the atrial cavity than in the ventricular cavity. Tumors within the heart can cause obstruction of the cardiac chambers and produce corresponding symptoms and signs. Extensive replacement of cardiac muscle by tumor tissue can lead to weakened myocardial contraction, resulting in heart failure. Infiltration of tumor cells into the cardiac conduction system can cause arrhythmias or blockages in the atrioventricular bundle or its branches, leading to sudden death. Involvement of the epicardium or pericardium by the tumor can result in hemopericardium and cardiac tamponade.
It is difficult to distinguish between malignant and benign heart tumors preoperatively; a correct pathological diagnosis is usually made postoperatively from the excised tumor specimen or during autopsy. Due to the difficulty in achieving complete radical resection of malignant heart tumors during surgery, there is a high chance of local tumor recurrence or distant metastasis to vital organs, leading to death.
Left atrial myxoma should first be differentiated from rheumatic heart disease with mitral valve lesions. A detailed medical history can provide some basis for differential diagnosis. For example, if there is no history of wind-dampness Rebing in this disease, a transient syncope history may be present. The course of the disease is generally short, and the condition progresses rapidly, especially when systemic embolism occurs under sinus rhythm and no other disease cause can be identified, the possibility of left atrial myxoma should be highly suspected. A short, non-progressive diastolic murmur in the apical region, especially if the nature and intensity of the murmur change with body position, can also serve as a reference for differential diagnosis. Echocardiography has special diagnostic value. To obtain a definitive diagnosis before surgery and avoid unnecessary surgical exploration, it is absolutely necessary to perform echocardiography before surgery for wind-dampness-related valve membrane lesions. Right atrial myxoma needs to be differentiated from tricuspid stenosis, tricuspid valve displacement, chronic constrictive pericarditis, and cardiomyopathy. In some cases, selective intracardiac angiography can reveal the size, location, and mobility of the tumor for differentiation.




