| disease | Pulmonary Embolism |
| alias | Pulmonary Embolism |
Pulmonary embolism refers to the pathological and clinical condition caused by embolic material entering the pulmonary artery and its branches, obstructing blood supply to the tissues. The most common emboli are thrombi, while others include rare neoplastic cells, fat droplets, air bubbles, drug particles from intravenous administration, or even catheter tips causing pulmonary vascular obstruction. Since the lung tissue receives dual blood supply from the bronchial artery and pulmonary artery, and gas exchange can occur directly between lung tissue and alveoli, most pulmonary embolisms do not necessarily lead to pulmonary infarction.
bubble_chart Epidemiology
The incidence of pulmonary embolism is high abroad. In the United States, the annual incidence is approximately 600,000 cases, with one-third resulting in death, making it the third leading cause of mortality. Some reports indicate that in recent years, as more adults receive anticoagulant therapy, the incidence has shown a decreasing trend. There is no precise epidemiological data in China, but a report from Fuwai Hospital on over 900 autopsy cases of cardiovascular and pulmonary diseases revealed that large thrombotic blockages in pulmonary segments or above accounted for 100 cases (11%), constituting 29% of autopsies in rheumatic heart disease, 26% in cardiomyopathy, and 19% in lung heart disease, indicating that pulmonary embolism frequently complicates cardiovascular and pulmonary diseases.
(1) Thrombosis Pulmonary embolism is often a complication of venous thrombosis. The emboli usually originate from the deep veins of the lower limbs and pelvis, traveling through the circulation to the pulmonary arteries, causing embolism. Rarely, they may originate from the veins of the upper limbs, head, and neck. Blood stasis, increased blood coagulability, and venous endothelial injury are promoting factors for thrombosis. Therefore, trauma, prolonged bed rest, varicose veins, venous catheterization, pelvic and hip surgery, obesity, diabetes, contraceptive use, or other causes of hypercoagulability can easily induce venous thrombosis. Early thrombi are fragile, and combined with the action of the fibrinolytic system, the risk of pulmonary embolism is highest in the first few days after thrombosis formation.
(2) Heart Disease This is the most common cause of pulmonary embolism in China, accounting for 40%. It is associated with various types of heart disease, with higher incidence rates in cases complicated by atrial fibrillation, heart failure, and subacute bacterial endocarditis. Right heart thrombi are the most common, while a few originate from the venous system. Bacterial emboli are seen not only in subacute bacterial endocarditis but also in pacemaker infections. In the former, infectious emboli mainly originate from the tricuspid valve, and occasionally, in congenital heart disease patients, mitral valve vegetations may pass from the left heart through a defect into the right heart and reach the pulmonary arteries.(3) Tumors In China, this is the second leading cause, accounting for 35%, significantly higher than the 6% reported abroad. Common malignancies include lung cancer, digestive system tumors, choriocarcinoma, and leukemia. Among malignant tumors complicated by pulmonary embolism, only about one-third are tumor emboli, while the rest are thrombi. It is speculated that tumor patients may have thrombin (thromboplastin) and other substances in their blood that activate the coagulation system, such as histones, cathepsins, and proteolytic enzymes. Therefore, the incidence of pulmonary embolism in tumor patients is high, and it may even be the first presenting symptom.
(4) Pregnancy and Childbirth The incidence of pulmonary embolism in pregnant women is several times higher than in non-pregnant women of the same age, with the highest rates occurring postpartum and after cesarean section. During pregnancy, increased intra-abdominal pressure, hormonal relaxation of vascular smooth muscle, and compression of pelvic veins can cause venous stasis, alter hemorheological properties, and exacerbate venous thrombosis. Additionally, there is an increase in clotting factors and platelets, along with reduced activity of the plasminogen-plasmin proteinolytic system. However, these changes do not show absolute differences compared to pregnant women without thromboembolism. Amniotic fluid embolism is also a serious complication during childbirth.
(5) Others Other rare causes include long bone fractures leading to fat embolism, accidents and decompression sickness causing air embolism, as well as parasitic and foreign body emboli. When no obvious precipitating factors are present, hereditary reductions in anticoagulant factors or increases in plasminogen activator inhibitors should also be considered.bubble_chart Pathological Changes
Most acute pulmonary embolisms can involve multiple pulmonary {|###|} arteries, with the right lung being more commonly affected than the left, and the lower lobes more than the upper lobes. However, embolisms rarely occur in the main {|###|} artery of the right or left lung or straddle the bifurcation of the pulmonary {|###|} artery. When thromboemboli are poorly organized, they are prone to fragment during passage through the heart, leading to embolisms in smaller vessels. If the fibrinolytic mechanism fails to completely dissolve the thrombus, the surface of the embolus begins to be covered by endothelial-like cells within 24 hours, firmly adhering to the {|###|} arterial wall within 2–3 weeks, followed by vascular remodeling. Early embolus retraction, the冲刷 effect of reestablished blood flow, fibrin and platelet aggregates covering the embolus surface, and the thrombolytic process can all generate new emboli, further occluding small vascular branches. Whether an embolus causes pulmonary infarction depends on the size of the affected vessel, the extent of occlusion, the ability of the bronchial {|###|} arteries to supply blood flow, and whether ventilation in the occluded area is adequate. The histological hallmark of pulmonary infarction is alveolar hemorrhage and alveolar wall necrosis, but inflammation is rarely observed. Cavitation is extremely rare unless there is preexisting pulmonary infection or the embolus is noninfectious. Loss of pulmonary surfactant in the infarcted area can lead to atelectasis, and pleural effusion is common, with one-third being bloody. If the patient survives, the infarcted area eventually forms a scar.
Pulmonary embolism increases physiological dead space and reduces ventilation efficiency. However, since acute pulmonary embolism can stimulate ventilation, increasing respiratory rate and minute ventilation, it usually offsets the increase in dead space, maintaining PaCO2 without elevation or even lowering it. Alveolar hyperventilation is unrelated to hypoxemia and cannot be alleviated by oxygen inhalation. The mechanism remains unclear but is speculated to involve reflexes from the lung parenchyma in the embolized vascular region. Although PaCO2 is typically reduced, CO2 retention may occur in patients with neuromuscular disorders, severe pleuritic pain, or severe pulmonary embolism who cannot compensate for the increased dead space by increasing ventilation. Acute pulmonary embolism often leads to decreased PaO2, with ventilation/perfusion mismatch being the primary mechanism. Local bronchoconstriction, atelectasis, and pulmonary edema serve as the anatomical basis. If cardiac output fails to meet metabolic demands, mixed venous oxygen tension decreases, further exacerbating ventilation/perfusion mismatch and hypoxemia.The hemodynamic response to pulmonary embolism is complex, involving both direct mechanical effects and chemical/reflex mechanisms post-embolism. Small or few emboli do not alter pulmonary hemodynamics. Generally, when >30% of the pulmonary vascular bed is obstructed, mean pulmonary {|###|} artery pressure begins to rise; when >35%, right atrial pressure increases; and when >50% is lost, significant increases in pulmonary {|###|} artery pressure and vascular resistance occur, along with reduced cardiac index and acute cor pulmonale. Recurrent pulmonary embolisms lead to persistent pulmonary {|###|} hypertension and chronic cor pulmonale. In patients with preexisting cardiopulmonary impairment, the hemodynamic impact of pulmonary embolism is far more pronounced than in normal individuals.
bubble_chart Clinical Manifestations
The clinical manifestations of pulmonary embolism can range from asymptomatic to sudden death. Common symptoms include dyspnea and chest pain, with an incidence rate of over 80% each. Pleuritic pain is caused by adjacent pleural fibrinous inflammation, and sudden onset often indicates pulmonary infarction. Involvement of the diaphragmatic pleura may radiate to the shoulder or abdomen. If there is retrosternal pain, it may resemble myocardial infarction. Chronic pulmonary infarction may present with hemoptysis. Other symptoms include anxiety, which may be caused by pain or hypoxemia. Syncope is often a sign of pulmonary infarction.
Common signs include tachypnea, cyanosis, pulmonary moist rales or wheezing, pulmonary vascular murmurs, pleural friction rub, or signs of pleural effusion. Circulatory system signs include tachycardia, accentuated P2, and manifestations of shock or acute/chronic cor pulmonale. Approximately 40% of patients have low to moderate fever, while a few may experience high fever in the early stages.
Approximately 20–30% of patients die due to delayed or missed diagnosis and treatment. If diagnosed early and treated with anticoagulants, the mortality rate can be reduced to 8%, highlighting the importance of early diagnosis. A thorough medical history should be collected. Elevated serum LDH, decreased stirred pulse blood PO2, and widened PA~aO2 are indicative. Electrocardiogram (ECG) may show T-wave and ST-segment changes (resembling myocardial infarction) or alterations in P-wave and QRS complex morphology (similar to acute lung heart disease). Chest X-ray may reveal patchy infiltrates, atelectasis, elevated diaphragm, pleural effusion, especially a rounded dense shadow with a convex border toward the hilum (Hampton’s hump), and dilated pulmonary stirred pulse with distal vascular pruning (Westermark’s sign), all of which are valuable for diagnosing pulmonary embolism. Radionuclide ventilation/perfusion (V/Q) scanning is the most sensitive non-invasive method for diagnosing pulmonary embolism. Although its specificity is low, the presence of typical multiple, segmental, or wedge-shaped perfusion defects with normal or increased ventilation, combined with clinical findings, confirms the diagnosis. Pulmonary stirred pulse angiography is the most specific diagnostic method, suitable for cases with clinical or radionuclide suspicion and those requiring surgical intervention. Findings include vascular filling defects, stirred pulse cutoff, or the "pruning sign." Angiography cannot detect vessels ≤2 mm in diameter, so small multiple emboli are often misdiagnosed as fistula disease. Magnetic resonance imaging (MRI) is a useful non-invasive technique for diagnosing pulmonary embolism, showing significant stirred pulse filling defects in larger emboli. (Figures 1–6)

Figure 1 Imaging features of pulmonary embolism
X-ray signs of left lower lobe pulmonary embolism
Left lower lobe infiltrate (posteroanterior view)
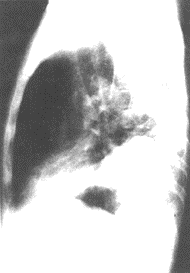
Figure 2 Imaging features of pulmonary embolism
X-ray signs of left lower lobe pulmonary embolism
Widened pleural membrane-based shadow (left lateral view)
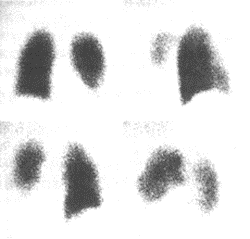
Figure 3 Imaging features of pulmonary embolism
Radionuclide
scan of left lower lobe pulmonary embolism
Ventilation scan shows no significant abnormalities 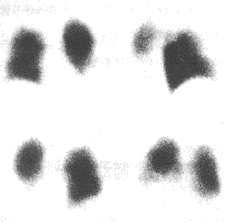
Figure 4 Imaging features of pulmonary embolism
Radionuclide scan of left lower lobe pulmonary embolism
Left lower lobe perfusion scan shows reduced radioactivity
(Mismatch suggests pulmonary embolism)
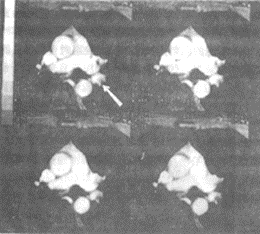
Figure 5 Imaging features of pulmonary embolism
MRI of left lower lobe pulmonary embolism
MRI cine, arrow indicates stirred pulse filling defect
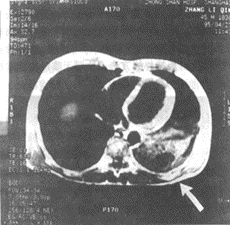
Figure 6 Imaging features of pulmonary embolism
MRI of left lower lobe pulmonary embolism
MRI cross-sectional image shows a large area of low signal intensity in the left lower lobe, suggesting infarction
Pulmonary embolism can mimic pneumonia, pleural membrane inflammation, pneumothorax, chronic obstructive pulmonary disease (COPD), lung tumors, coronary artery disease, acute myocardial infarction, congestive heart failure, cholecystitis, pancreatitis, and other conditions, requiring careful differentiation.
bubble_chart Treatment Measures
In addition to symptomatic treatments such as oxygen inhalation, pain relief, correction of shock and heart failure, and bronchodilation, specific methods include anticoagulation, thrombolysis, and surgical intervention, with the treatment protocol illustrated in Figure 7.
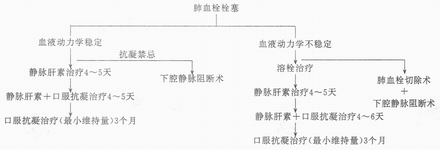
Figure 7 Treatment of Pulmonary Embolism
For anticoagulation and thrombolysis therapies, refer to "Myocardial Infarction." Inferior vena cava interruption is suitable for patients at risk of fatal bleeding during anticoagulation therapy or those with recurrent embolism, which can be achieved through ligation, placement of specialized clips, or filters. Pulmonary thrombectomy carries a high mortality rate and is reserved only for patients whose shock persists despite aggressive thrombolytic or vasopressor therapy.




