| disease | Complete Transposition of the Great Arteries |
Complete transposition of the great arteries (TGA) refers to the abnormal switching of the positions of the two major arteries. The aorta receives deoxygenated blood from the right ventricle of the systemic venous circulation, while the pulmonary artery receives oxygenated blood from the left ventricle via the pulmonary veins, thus forming two separate circulatory systems. One system follows the path: right atrium → right ventricle → aorta → body → systemic veins → right atrium. The other follows: left atrium → left ventricle → pulmonary artery → lungs → pulmonary veins → left atrium. When the ventricular positions are normal but the aorta originates to the right of the pulmonary artery, it is termed dextro-transposition of the great arteries (D-TGA). D-TGA is the most common clinical type and is often associated with atrial septal defect, ventricular septal defect, patent ductus arteriosus, pulmonary artery stenosis, and atrioventricular canal defects.
bubble_chart Clinical Manifestations
Clinical Classification: Based on the presence of concomitant cardiovascular anomalies, this condition can be divided into four types:
Type I: Intact ventricular septum but accompanied by atrial septal defect or patent foramen ovale (possibly with patent ductus arteriosus), accounting for approximately 50%.
Type II: Accompanied by ventricular septal defect (possibly with patent foramen ovale or patent ductus arteriosus), accounting for about 25%.
Type III: Accompanied by pulmonary stenosis and/or ventricular septal defect (possibly with patent foramen ovale or patent ductus arteriosus), accounting for around 10%.
Type IV: Accompanied by ventricular septal defect and pulmonary vascular obstructive disease or other anomalies, accounting for approximately 15%.
Clinical Manifestations: Primarily include dyspnea, cyanosis, progressive cardiac enlargement, and early-onset heart failure. Depending on the type of lesion, the degree of pulmonary congestion and the volume of blood shunting between systemic and pulmonary circulation vary, leading to differences in symptoms and their timing of onset.Type I: Hypoxia, cyanosis, tachypnea, acidosis, and heart failure appear at birth or within a few days. A systolic ejection murmur may be heard. Death often occurs within days due to severe hypoxemia.
Type II: Symptoms appear later, with tachypnea, cyanosis, and congestive heart failure developing within weeks or months after birth. In cases with a large ventricular septal defect, significant shunting between systemic and pulmonary circulation occurs, leading to cardiac enlargement, hepatomegaly, and a harsh pansystolic or systolic ejection murmur typically heard at the lower left sternal border.
Type III: Cases with pulmonary valve, annulus, or subvalvular stenosis exhibit reduced pulmonary blood flow, delayed onset of pulmonary hypertension and pulmonary vascular obstructive disease, and later symptom presentation. The clinical presentation resembles that of tetralogy of Fallot, with cyanosis, hypoxia, and acidosis, but heart failure is rare.
Type IV: Generally, after one year of age, pulmonary vascular obstructive disease develops due to pulmonary hypertension, manifesting as dyspnea, heart failure, and progressive cyanosis. In addition to a systolic murmur, the second heart sound of the pulmonary valve is often accentuated.
bubble_chart Auxiliary Examination
Chest X-ray examination: At birth, the heart size is normal, but it gradually enlarges over time, with increased pulmonary vascular markings. The cardiac silhouette appears as an obliquely positioned egg shape. Due to the overlap of the main {|###|} artery and pulmonary {|###|} artery shadows, the upper mediastinal cardiac pedicle appears narrow. Unless accompanied by pulmonary {|###|} artery stenosis, pulmonary vascular markings are generally increased. In cases of large ventricular septal defects with pulmonary {|###|} artery hypertension, the heart is significantly enlarged, pulmonary vascular markings are increased, and pulmonary edema may be present.
Electrocardiogram (ECG) examination: Right axis deviation and right ventricular hypertrophy are observed. If accompanied by a ventricular septal defect or patent {|###|} ductus arteriosus, there may be biventricular hypertrophy and myocardial damage.Echocardiogram examination: A horizontal cross-section at the level of the main {|###|} artery root shows the pulmonary {|###|} artery located posteriorly and to the left, while the main {|###|} artery is located anteriorly and to the right. The pulmonary {|###|} artery, originating from the left ventricle, divides into left and right branches, whereas the main {|###|} artery originates from the right ventricle. The pulmonary {|###|} valve opens earlier and closes later than the main {|###|} valve.
Right heart catheterization: A catheter is inserted via the femoral vein into the right atrium, right ventricle, and then into the main {|###|} artery. Alternatively, it may pass through a patent foramen ovale in the right atrium to enter the left atrium {|###|} transmission from one meridian to the next left ventricle and then the pulmonary {|###|} artery. The right ventricular systolic pressure approximates systemic circulation pressure, and the oxygen saturation in the main {|###|} artery is low.
Right ventricular angiography: The main {|###|} artery is immediately visualized. If a ventricular septal defect is present, its size and location can be demonstrated, and the left ventricle and pulmonary {|###|} artery will also be visualized simultaneously.
(1) Tetralogy of Fallot Pulmonary {|###|} artery pulse The second heart sound is weakened, X-ray shows pulmonary ischemia, and the heart shadow is enlarged in a boot shape. Large {|###|} displaced pulmonary {|###|} artery pulse The second heart sound is normal or hyperactive, pulmonary vascularity is increased, and the heart is enlarged. Echocardiography and cardiac {|###|} angiography can confirm the diagnosis.
(2) Persistent {|###|} truncus arteriosus Echocardiography shows the {|###|} truncus arteriosus straddling the ventricular septum. Right heart catheterization reveals equal pressures in the left and right ventricles. Cardiac {|###|} angiography shows a single {|###|} truncus arteriosus located above the ventricular septum, with the coronary {|###|} artery and pulmonary {|###|} artery both originating from the {|###|} truncus arteriosus.bubble_chart Treatment Measures
(1) Medical Treatment Once a newborn is diagnosed, prostaglandin E1 should be administered intravenously immediately at a dose of 0.1 μg/(kg·min). If effective, it can be maintained for 24 hours or several days to keep the ductus arteriosus open, increase oxygen saturation, and reduce cyanosis. Additionally, heart failure should be controlled, and hypoxia and acidosis should be corrected to create conditions for further treatment.
(2) Surgical Treatment
Surgical Indications
(1) If severe cyanosis and heart failure are present at birth and the infant cannot tolerate corrective surgery, emergency balloon atrial septostomy can be performed. If the procedure fails, cyanosis does not improve, oxygen saturation remains unsatisfactory, and heart failure persists, partial atrial septectomy may be performed.
(2) For transposition of the great arteries (TGA) with ventricular septal defect, if medical treatment fails to control congestive heart failure, pulmonary artery banding should be performed within 1–2 days after birth.
(3) For TGA with pulmonary stenosis, a systemic-to-pulmonary artery shunt should be performed.
(4) If the child survives to 6 months–1 year of age, corrective surgery can be performed.
(3) Palliative Surgical Methods
1. Balloon Atrial Septostomy (Rashkind Procedure) When TGA is suspected in a newborn, a balloon catheter is inserted into the right ventricle for angiography. After confirmation of the diagnosis, the catheter is withdrawn into the right atrium, passed through the foramen ovale into the left atrium, and confirmed by pressure or oxygen measurement. Then, 1.5–2.0 mL of contrast agent is injected to inflate the balloon, which is quickly pulled back into the right atrium or inferior vena cava. This is repeated 2–3 times to ensure adequate tearing of the atrial septum. A successful outcome includes increased oxygen saturation, corrected acidosis, and elimination of the pressure gradient between the left and right atria. Symptoms typically improve until around 1 year of age, so corrective surgery is recommended at 6 months–1 year. Common complications include cardiac perforation, tricuspid valve or inferior vena cava laceration, with a surgical mortality rate of about 5%.
2. Partial Atrial Septectomy (Blalock-Hanlon Procedure) If the Rashkind procedure does not provide sufficient relief and cyanosis worsens, a closed surgical approach can be used to remove part of the right edge of the atrial septum, artificially creating a larger atrial septal defect. This often provides adequate mixing of blood between the left and right atria to alleviate symptoms and is commonly used in young children.
3. Systemic-to-Pulmonary Artery Shunt (Shunt Procedure) This includes various systemic-to-pulmonary artery anastomoses and is suitable for cases of TGA with pulmonary stenosis. It effectively improves hypoxia, is simple to perform, and is suitable for young children. However, if the anastomosis is too large, excessive blood flow into the pulmonary circulation may cause heart failure.
4. Pulmonary Artery Banding (Banding Procedure) This is suitable for infants with TGA and excessive pulmonary blood flow leading to congestive heart failure who are not candidates for corrective surgery. A band is placed around the main pulmonary artery, constricting it by about 50–60%. The band length is 24 mm plus the patient's weight in kilograms (mm). The target pressure gradient across the band is 5.332 kPa (40 mmHg), with pulmonary artery pressure reduced by one-third and right ventricular pressure increased by one-quarter compared to pre-banding levels. The distal pulmonary artery pressure should drop to one-third to one-half of the aortic pressure, with a slight decrease in left atrial pressure and a slight increase in aortic pressure. Postoperatively, the goal is to reduce left-to-right intracardiac shunting and pulmonary blood flow, thereby decreasing pressure on the pulmonary vascular bed and preparing for corrective surgery. The main complication is right ventricular outflow tract or pulmonary artery obstruction leading to right heart failure.
(4) Corrective Surgical Methods
1. Intra-atrial Baffle Procedure (Mustard Operation) A barrier is constructed within the right atrium using pericardium or Dacron fabric, positioned around the superior and inferior vena cava. This directs the venous blood from the systemic circulation (i.e., blood from the vena cava) toward the tricuspid valve and into the posterior left ventricle, which then pumps it to the lungs. Meanwhile, pulmonary venous blood is directed toward the tricuspid valve and into the anterior right ventricle, which pumps it to the aorta. Although this anatomically complicates the malformation further, it achieves hemodynamic physiological function. Complications may include obstruction of the vena cava or pulmonary veins, arrhythmias, chronic heart failure, and tricuspid valve insufficiency (Figure 1).
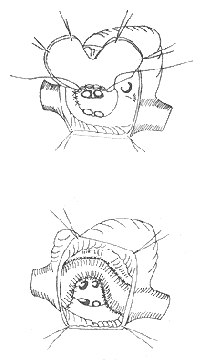
Figure 1 Schematic diagram of the Mustard procedure
2. Senning Procedure This technique uses atrial septal tissue and atrial walls to create intra- and extra-cardiac tunnels to redirect venous blood flow. The key difference from the Mustard procedure is that it requires only a small patch to form the intracardiac tunnel, which helps preserve atrial growth potential. Unlike the Mustard procedure, where blood flows at the atrial septal level, the Senning procedure uses an extracardiac pathway, eliminating the need for precise patch design. Postoperative atrial function remains unaffected, and caval or pulmonary venous obstruction is rare. Complications include arrhythmias and heart failure (Figure 2).
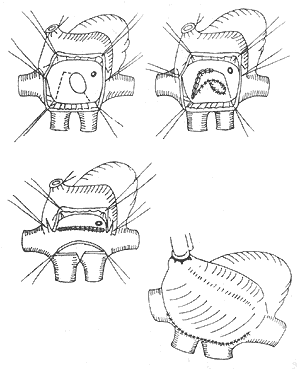
Figure 2 Schematic diagram of the Senning procedure
3. Rastelli Procedure This involves using a valved extracardiac conduit to reestablish continuity between the right ventricle and the pulmonary artery, thereby correcting severe obstruction or even complete discontinuity between the right ventricle and the pulmonary artery. Complications include calcification, dysfunction, or obstruction of the conduit valve, as well as bleeding and heart failure (Figure 3).
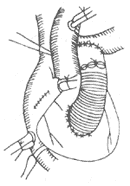
Figure 3 Schematic diagram of the Rastelli procedure
4. Anatomical Correction of the Great Arteries (Switch Procedure) This ideal and physiologically rational procedure involves relocating the aorta to the left ventricle and the pulmonary artery to the right ventricle. However, it requires coronary artery transplantation and is technically demanding. Complications include heart failure and myocardial ischemia due to coronary artery ostial stenosis (Figure 4).
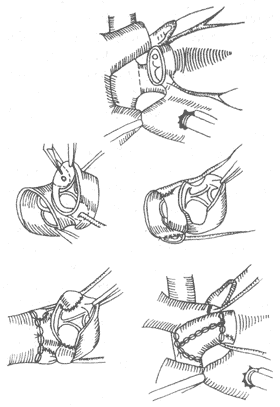
Figure 4 Schematic diagram of the Switch procedure
5. Damas-Kaye-Stanel Procedure This technique avoids coronary artery transplantation. The main pulmonary artery is transected at its bifurcation, and the proximal end is trimmed into a beveled opening. The aorta is incised posterolaterally and anastomosed end-to-side to the proximal pulmonary artery. The ventricular septal defect is repaired via a right ventricular outflow tract incision. The aortic valve annulus is closed with a Dacron patch to seal the right ventricular outflow tract, and a valved conduit is placed between the right ventricle and the distal pulmonary artery. Complications include calcification, dysfunction, or obstruction of the valved conduit, as well as heart failure.
Complete transposition of the great arteries (TGA) without ventricular septal defect has a poor prognosis, with approximately 80–90% of cases dying within the first year of life. Without surgery, about 45% die within one month, 69% within three months, 75% within eight months, and 80% within the first year. In recent years, due to the development of pediatric cardiac surgery in China and the emphasis on the diagnosis and treatment of TGA in pediatric cardiology, the survival rate of infants after birth has increased, creating conditions for radical cardiac surgery. Common causes of death without surgery include heart failure, pulmonary infection, hypoxia, cerebral hemorrhage, and cerebral embolism caused by polycythemia. Postoperative common causes include heart failure, low cardiac output syndrome, respiratory failure, and complete atrioventricular block.




