| disease | Tuberculosis |
Subcutaneous node disease is a chronic pestilence caused by subcutaneous node mycobacteria, which can affect many organs, with pulmonary tuberculosis (pulmonary subcutaneous node) being the most common. Patients who shed bacteria are an important source of pestilence. Infection with subcutaneous node bacteria does not necessarily lead to illness; clinical symptoms may only appear when resistance is weakened or cell-mediated hypersensitivity increases. The basic pathological features of this disease include exudation, caseous necrosis, and other proliferative tissue reactions, which may lead to cavity formation. Except for a few cases with acute onset, the clinical course is mostly chronic, presenting with systemic symptoms such as low-grade fever, weight loss, and lack of strength, as well as respiratory manifestations like cough and hemoptysis. If diagnosed promptly and treated appropriately, most cases can achieve clinical recovery. Since the 1950s, although the prevalence of subcutaneous node disease in China has declined, control efforts remain uneven across regions, making it a prominent public health issue and one of the top ten causes of death nationwide.
bubble_chart Epidemiology
Subcutaneous node disease is one of the oldest pestilence diseases in human history, with humanity battling it for thousands of years. Yet, to this day, subcutaneous node disease remains prevalent worldwide, posing a severe threat to human health and life. Currently, due to the emergence and spread of drug-resistant subcutaneous node bacteria, co-infections of subcutaneous node bacteria with the human immunodeficiency virus (HIV), and inadequate control measures in many countries, the global incidence of subcutaneous node disease is showing a significant upward trend. Among all pestilence-related diseases worldwide, subcutaneous node disease has become the leading cause of death among adults. The annual global death toll from subcutaneous node disease exceeds the combined deaths from Acquired Immune Deficiency Syndrome, malaria, diarrhea, and tropical diseases, and it claims the lives of 300,000 children. According to a WHO bulletin, over 7 million new cases of subcutaneous node disease were reported worldwide in 1997, with nearly 3 million deaths attributed to the disease.
The increasing resistance of subcutaneous node bacteria to medicinal properties is a major factor contributing to the disease's potential resurgence as a refractory condition and the rise in outbreaks. Chemical drugs remain the most powerful weapon for controlling subcutaneous node disease, with over 95% of newly diagnosed patients achieving cure rates through proper medication, while also preventing the development of drug resistance. However, improper drug use or poor management—such as irregular medication adherence by patients—can lead to treatment failure, resulting in drug-resistant cases and the further spread of multidrug-resistant subcutaneous node bacteria. For patients infected with drug-resistant subcutaneous node bacteria, chemical drugs often prove ineffective.Additionally, it is estimated that there are currently 50 million refugees and migrants worldwide, half of whom are already infected with subcutaneous node bacteria. Due to their mobile and precarious living conditions, they often struggle to receive proper treatment once the disease manifests, contributing to the spread of subcutaneous node disease, particularly drug-resistant strains. The WHO estimates that at least two-thirds of patients globally are now at risk of developing multidrug resistance. The high resistance rates and the continuous spread of multidrug-resistant subcutaneous node bacteria will make the disease increasingly difficult to control with existing chemotherapy.
The rapidly growing global trend of subcutaneous node disease outbreaks presents a severe challenge to international public health. In response, the WHO declared in April 1993 that the world was in a state of subcutaneous node disease emergency. This unprecedented move aimed to urgently draw the attention of governments and international organizations to the critical need for controlling the disease's spread.
The cause of pulmonary subcutaneous node disease is clear, with preventive measures and treatment methods available. However, only by rigorously addressing every aspect—treatment, management, prevention, and screening—and ensuring that every diagnosed case is treated thoroughly can the epidemic situation of subcutaneous node disease be improved and ultimately brought under control.
1. Subcutaneous node bacteria
belong to the order Actinomycetales and the genus Mycobacterium of the family Mycobacteriaceae. The primary causative agent of human subcutaneous node disease is the human-type subcutaneous node bacterium, while bovine-type infections are rare. Subcutaneous node bacteria are aerobic, difficult to stain, and retain their color even after being washed with acidic alcohol following heating with fuchsin, hence they are called acid-fast bacilli. Microscopically, they appear as slender, slightly curved rods. They exhibit strong resistance to external environments, surviving for over five months in damp, shady conditions. However, they can be killed by two hours of direct sunlight, exposure to 5–12% cresol soap (lysol) solution for 2–12 hours, contact with 70% alcohol for two minutes, or boiling for one minute. The simplest sterilization method is to directly incinerate sputum-contaminated paper. Subcutaneous node bacteria grow slowly, requiring 15–20 hours to reproduce a generation, and typically take 4–6 weeks (at least three weeks) to form visible colonies.
Lesions often contain populations of subcutaneous node bacteria with varying growth rates (Figure 1). Group A: Rapidly growing and proliferating, extracellular, highly pathogenic, and highly infectious, predominantly found in early-stage active lesions, cavity walls, or cavities. They are easily killed by anti-subcutaneous node drugs, particularly isoniazid, which is the most effective bactericidal agent, followed by streptomycin and rifampin, though these are less effective than isoniazid. Group B: Intracellular bacteria residing within macrophages, protected by the acidic cytoplasm, allowing survival but slow reproduction. Pyrazinamide is effective at pH <5.5時,殺菌效果較好。C群:為偶爾繁殖菌,存在於乾酪壞死灶內,生長環境對細菌不利,結核菌常呈休眠狀態,僅偶爾發生短暫的生長繁殖,僅對少數藥物如利福平敏感。B群與C群菌為頑固菌,常為日後復發的根源,僅暫時休眠,可能存活數月、數年。亦稱 "持續存活菌" 。D群:為休眠菌,病灶中有少量結核菌完全處於休眠狀態,無致病力及傳染性,對人體無害。任何藥物對其作用,多數自然死亡或被吞噬殺滅,很少復發。

Figure 1: Schematic diagram of bacterial populations with different growth rates in lesions and the effects of bactericidal drugs
The above classification based on bacterial growth and reproduction provides certain guidance for drug selection.
During the reproduction process, subcutaneous node bacteria develop resistance to medicinal properties due to chromosomal gene mutations. Resistance to medicinal properties is an important biological characteristic of subcutaneous node bacteria, which determines the success or failure of treatment. Naturally resistant bacteria continue to grow and reproduce, eventually becoming the dominant strain in the bacterial population (as sensitive bacteria are eliminated by the drugs), rendering anti-subcutaneous node drugs ineffective. Such naturally resistant bacteria (natural variants) that arise in very small quantities due to gene mutations usually do not cause serious consequences. Another mechanism for developing resistance to medicinal properties occurs when drugs come into contact with subcutaneous node bacteria, inducing some bacteria to undergo adaptive mutations, gradually enabling them to survive in drug-containing environments (acquired resistance). On solid culture media, subcutaneous node bacteria that can grow in the presence of 1μg/mL isoniazid (INH), 10μg/mL streptomycin (SM), or 50μg/mL rifampicin (RFP) are referred to as resistant strains to the respective drugs. INH-resistant strains exhibit significantly reduced pathogenicity in animals, SM-resistant bacteria generally show no decrease in pathogenicity, and RFP-resistant bacteria display varying degrees of reduced pathogenicity. Subcutaneous node bacteria resistant to both RFP and INH exhibit a more pronounced decrease in pathogenicity compared to those resistant to INH alone.
Patients who have never used a certain drug before but whose sputum bacteria are resistant to that drug are said to have primary drug-resistant bacterial infections. Long-term irrational drug use, through elimination or induction mechanisms, leads to the emergence of resistant bacteria, known as secondary drug resistance. Many retreatment cases involve secondary drug resistance. In recent years, the number of multidrug-resistant subcutaneous node bacteria has been increasing, becoming clinically difficult to cure. Any errors in drug combination, insufficient drug dose, irregular medication, interrupted treatment, or premature discontinuation can lead to bacterial resistance. The consequence of drug resistance is inevitably short-term treatment failure or long-term relapse. Therefore, avoiding and overcoming bacterial resistance is the key to successful chemotherapy for subcutaneous node disease.
About 5% of clinically positive sputum cultures are non-subcutaneous node mycobacteria (mycobacteria other than subcutaneous node mycobacteria and leprosy mycobacteria), which are also acid-fast bacteria widely found in nature. When the body's immunity is compromised, they can cause pulmonary and extrapulmonary infections, with clinical manifestations resembling subcutaneous node disease, but most are resistant to anti-subcutaneous node drugs. These non-subcutaneous node mycobacteria have biological characteristics different from subcutaneous node bacteria, such as growth at 28°C, smooth colonies, negative niacin tests, positive drug resistance contact tests, and no pathogenicity to guinea pigs.
II. Routes of Infection
Respiratory infection is the primary route for pulmonary subcutaneous node transmission, with droplet infection being the most common mode. The main source of contagion is the sputum of pulmonary subcutaneous node patients, especially those with smear-positive sputum who are untreated. Healthy individuals become infected by inhaling droplets expelled when patients cough or sneeze. Sputum droplets smaller than 10μg can enter the alveolar cavity or remain suspended in the air for extended periods due to their light weight. In poorly ventilated indoor environments, these infectious droplets can also be inhaled, causing infection. A secondary route of infection is through the digestive tract. Small amounts of weak or less virulent subcutaneous node bacteria are often eliminated by the body's immune defense mechanisms. Only when a large number of highly virulent subcutaneous node bacteria invade and the body's immunity is insufficient does infection lead to disease. Other rare routes of infection include the skin and urogenital system.
III. Human Reactivity
(1) Immunity and Allergic Reactions
The body's natural immunity (innate immunity) to subcutaneous node bacteria is nonspecific. Immunity acquired through BCG vaccination or subcutaneous node bacterial infection (acquired immunity) is specific, capable of killing or tightly surrounding invading subcutaneous node bacteria, preventing their spread, and promoting lesion healing. Acquired immunity is significantly stronger than natural immunity, but both provide relative protection against subcutaneous node disease. After infection with subcutaneous node bacteria, individuals with immunity do not develop subcutaneous node disease. Physical exercise helps enhance immunity; conversely, measles, diabetes, silicosis, Acquired Immune Deficiency Syndrome, and other chronic diseases, malnutrition, or the use of glucocorticoids and immunosuppressants weaken immune function, making individuals more susceptible to subcutaneous node bacterial infection and disease or reactivating previously stable lesions. Age affects natural resistance to subcutaneous node infection, with the elderly and young children being more susceptible due to lower cellular immunity in the elderly and immature cellular immune systems in children.
The immunity in subcutaneous node disease is primarily cellular immunity, manifested as lymphocyte sensitization and enhanced phagocyte function. After invading subcutaneous node bacteria are engulfed by phagocytes, the antigenic information is processed and transmitted to T lymphocytes, sensitizing them. When sensitized T lymphocytes re-encounter subcutaneous node bacteria, they release various lymphokines (including chemotactic factors, macrophage migration inhibitory factors, and macrophage activation factors), causing macrophages to gather around the bacteria, phagocytize, and kill them. These macrophages then transform into epithelioid cells and Langhans giant cells, ultimately forming subcutaneous node nodules, localizing the lesions.
After 4-8 weeks of the bacteria invading the human body, the sensitive reaction of body tissues to the bacteria and its metabolic products is called an allergic reaction, which is related to inflammatory mediators, skin reactive factors, and lymphotoxins released by another subgroup of T lymphocytes. Local inflammatory exudation or even caseous necrosis occurs, often accompanied by systemic symptoms such as fever, lack of strength, and loss of appetite. At this time, if a skin test is performed with the bacterial extract (details below), a positive reaction may occur. The injected local tissue becomes congested and edematous, with a large number of sensitized T lymphocytes infiltrating. This cellular immune response of the human body to the bacteria and its metabolic products belongs to type IV (delayed-type) hypersensitivity. After infection with the bacteria, skin manifestations such as erythema nodosum, polyarthritis, or herpetic conjunctivitis may also occur, all of which are manifestations of the allergic reaction to the bacterial disease, often seen in patients with primary bacterial infection.
The polypeptides and polysaccharide complexes of the subcutaneous node bacterium are related to the immune response, while its wax and subcutaneous node proteins are associated with allergic reactions. The antigenic components that cause these two responses differ, but immunity and allergic reactions often coexist. For example, after receiving the BCG vaccine, immunity is generated, and the subcutaneous node tuberculin reaction (allergic reaction) also turns positive. The occurrence of both may also be related to the lymphokines produced by different T lymphocyte subsets in the body. Immunity plays a protective role in humans, whereas allergic reactions are usually accompanied by tissue damage and are also detrimental to the bacteria. Severe illness, malnutrition, or the use of immunosuppressive drugs can weaken immunity, and allergic reactions are simultaneously suppressed, manifesting as a lack of response to subcutaneous node bacterial tests. When overall health improves or immunosuppressive drugs are discontinued, the subcutaneous node tuberculin reaction becomes positive again as immunity and allergic reactions recover. Immunity and allergic reactions sometimes do not align perfectly, influenced by the complex internal and external environment of the human body, the effects of drugs, as well as the quantity and virulence of the infecting bacteria. In summary, the occurrence, progression, and outcome of subcutaneous node disease after infection are determined by the quantity and virulence of the invading subcutaneous node bacteria and the level of human immunity and allergic reactions. When the body's resistance is compromised, subcutaneous node disease often progresses more easily; conversely, infection is less likely to lead to illness, and even if it does, the disease is usually milder and more easily cured.
(II) Primary Infection and Reinfection
When guinea pigs are inoculated with a certain amount of subcutaneous node bacteria for the first time, there may be no obvious reaction in the first few days. After about 10–14 days, the injection site becomes red and swollen, gradually forming an ulcer that does not heal easily. The subcutaneous node bacteria multiply extensively, spread to local lymph nodes, and disseminate throughout the body via the lymphatic and circulatory systems. The guinea pigs are prone to death, indicating that they lack immunity to subcutaneous node bacteria.
If the same amount of subcutaneous node bacteria is injected into guinea pigs that were infected with a small amount of subcutaneous node bacteria 4–6 weeks earlier, the resulting reaction is markedly different from the above. After injection, the animals develop a high fever, and within 2–3 days, the injection site shows severe reactions such as tissue redness, swelling, ulceration, and necrosis. However, the wound soon heals, scabs over, and the local lymph nodes do not swell. There is no systemic dissemination of subcutaneous node bacteria, and the animals do not die. This intense local allergic reaction caused by reinfection typically heals easily without systemic dissemination, all of which are results of the guinea pigs having developed immunity to subcutaneous node bacteria. The phenomenon of the body exhibiting different reactions to reinfection and primary infection with subcutaneous node bacteria is called the Koch phenomenon.
After the lungs are first infected with subcutaneous node bacteria (often in children, i.e., primary infection), the bacteria are carried by phagocytes to the hilar lymph nodes (causing lymph node swelling) and may disseminate systemically (occult bacteremia). If the body's immunity is low at this time, primary progressive subcutaneous node disease may develop. However, in adults (who often had grade I subcutaneous node infection in childhood or have been vaccinated with BCG) with established immunity, reinfection at this stage usually does not cause local lymph node swelling or systemic dissemination. Instead, a severe local tissue reaction occurs at the reinfection site, often with exudative lesions, or even caseous necrosis and dissolution, leading to cavity formation.
bubble_chart Pathological Changes
1. Basic pathological changes of subcutaneous node disease
Human immunity and allergic reactivity, the quantity and virulence of subcutaneous node bacteria invasion, are closely related to the nature and extent of subcutaneous node lesions, as well as the possibility and speed of transformation from one pathological type to another. Therefore, the pathological process is quite complex, and the basic pathological changes may not all appear in the lungs of subcutaneous node patients.
(1) Exudative lesions
Manifested as congestion, edema, and leukocyte infiltration. In the early stages of exudative sexually transmitted disease changes, neutrophils are present, which are gradually replaced by monocytes (phagocytes). Ingested subcutaneous node bacteria can be observed within large mononuclear cells. Exudative sexually transmitted disease changes typically appear in the early stages of subcutaneous node inflammation or during lesion exacerbation and can also be seen in serous membrane subcutaneous node. When the condition improves, exudative sexually transmitted disease changes can completely dissipate and be absorbed.
(2) Proliferative lesions
Initially, there may be a brief exudative phase. After large mononuclear cells phagocytize and digest subcutaneous node bacteria, the phospholipid components of the bacteria cause the large mononuclear cells to become enlarged and flattened, resembling epithelial cells, hence termed "epithelioid cells." Epithelioid cells aggregate into clusters, with Langhans giant cells potentially appearing in the center. The latter can transmit information about subcutaneous node bacterial antigens to lymphocytes, often surrounded by numerous lymphocytes, forming typical subcutaneous node nodules, which are characteristic sexually transmitted disease changes of subcutaneous node disease. This is also how "subcutaneous node" got its name. Subcutaneous node bacteria are usually difficult to find within subcutaneous node nodules. Proliferative lesions mostly occur when bacterial load is low and human cell-mediated immunity dominates.
(3) Degenerative lesions (caseous necrosis)
Often occur on the basis of exudative or proliferative sexually transmitted disease changes. If the body's resistance decreases, bacterial load is excessive, or allergic reactions are intense, subcutaneous node bacteria in exudative sexually transmitted disease changes overcome macrophages, continue to proliferate, cause cells to become cloudy and swollen, undergo fatty degeneration, dissolve, and fragment until cell necrosis occurs. After inflammatory cells die, they release proteolytic enzymes, leading to tissue dissolution and necrosis, forming coagulative necrosis. Due to the high lipid content, the lesion appears yellowish-gray to the naked eye, soft and brittle, resembling cheese, hence the name caseous necrosis. Microscopic examination reveals a coagulated, eosin-stained, non-subcutaneous node necrotic tissue.
The above three types of lesions can coexist within a single pulmonary lesion, but usually one is predominant. For example, small amounts of caseous necrosis may appear in the center of exudative and proliferative sexually transmitted disease changes; while degenerative lesions are often accompanied by varying degrees of exudation and subcutaneous node nodule formation.
2. Outcome of subcutaneous node lesions
In caseous necrosis lesions, the massive proliferation of subcutaneous node bacteria causes liquefaction, associated with neutrophil and large mononuclear cell infiltration. Part of the liquefied caseous necrotic material can be absorbed, while another part is expelled via the bronchi, forming cavities or causing bronchial dissemination within the lungs. When human immunity strengthens and anti-subcutaneous node drug treatment is administered, lesions can gradually heal. Exudative sexually transmitted disease foci can be absorbed and dissipated through the phagocytic action of the mononuclear-phagocyte system, leaving no scars. Smaller caseous necrosis or proliferative sexually transmitted disease changes can also shrink and be absorbed after treatment, leaving only slight fibrous scars. Lesions often accompany fibrous tissue proliferation during the healing process, forming cord-like scars. Caseous lesions can also dehydrate, shrink, and undergo calcium salt deposition, eventually forming calcified foci and healing.
3. Dissemination and exacerbation of subcutaneous node lesions
When the human body is first infected with subcutaneous node bacteria, the subcutaneous node bacteria can be phagocytized by cells and transported to the hilar lymph nodes via lymphatic vessels. A small amount of subcutaneous node bacteria may enter the bloodstream and disseminate throughout the body, but there may be no significant clinical symptoms (occult bacteremia). If necrotic lesions erode blood vessels, subcutaneous node bacteria can spread through the bloodstream, causing systemic foxtail millet-sized subcutaneous node infections, including those in the lungs, such as brain membrane, bone, and kidney subcutaneous node, among others. Subcutaneous node bacteria in the lungs can spread along the bronchi, forming new subcutaneous node lesions in other parts of the lungs. Ingesting large amounts of sputum containing subcutaneous node bacteria into the gastrointestinal tract can also lead to intestinal binding node, abdominal membrane subcutaneous node, and others. Pulmonary subcutaneous node can directly extend to the chest membrane, causing subcutaneous node-related chest membrane inflammation.
The pathological changes of subcutaneous nodules are related to the systemic immune function and the strength of local pulmonary immunity. Fibrosis is a manifestation of strong immunity, while cavity formation often indicates weakened immunity.
bubble_chart Clinical Manifestations
Typical pulmonary tuberculosis has an insidious onset and a prolonged course, with symptoms such as low-grade fever, fatigue, loss of appetite, cough, and slight hemoptysis. However, most patients have mild lesions with no significant symptoms and are occasionally discovered during routine X-ray health examinations. Some are diagnosed only after sudden hemoptysis, and a review of their medical history may reveal mild systemic symptoms. A small number of patients present with sudden onset and prominent toxic symptoms along with respiratory symptoms, leading to X-ray confirmation of acute miliary tuberculosis or caseous pneumonia. In elderly patients with pulmonary tuberculosis, symptoms are often masked by chronic bronchitis. Occasionally, severe undetected tuberculosis may lead to high fever due to secondary infection, or even progress to sepsis or respiratory failure before medical attention is sought. Given the diverse clinical manifestations of pulmonary tuberculosis, healthcare workers in regions where tuberculosis is largely under control and incidence is low should remain vigilant for atypical presentations in daily clinical practice.
Symptoms
(1) Systemic symptoms
These include afternoon low-grade fever, lack of strength, decreased appetite, weight loss, and night sweats. If the pulmonary lesions progress or disseminate, irregular high fever may occur. Women may experience menstrual disorders or amenorrhea.
(2) Respiratory symptoms
Typically, patients present with a dry cough or small amounts of mucoid sputum. In cases of secondary infection, the sputum becomes mucopurulent. About one-third of patients experience varying degrees of hemoptysis. Blood-streaked sputum is often due to capillary dilation in inflamed lesions, while moderate to severe hemoptysis is related to small vessel injury or rupture of aneurysms from cavities. Low-grade fever often follows hemoptysis, possibly due to residual blood clots in small bronchi being absorbed or infection caused by bronchial obstruction. Persistent fever suggests dissemination of tuberculosis lesions. Occasionally, calcified tuberculous lesions may cause hemoptysis due to mechanical vascular injury or complicating bronchiectasis. Massive hemoptysis can lead to hemorrhagic shock, and in rare cases, airway obstruction by blood clots may cause asphyxia, marked by extreme dysphoria, anxiety, restlessness, chest tightness, dyspnea, and cyanosis, requiring immediate emergency intervention.
When inflammation involves the parietal pleura, stabbing pain may occur in the corresponding chest wall, usually mild but worsening with breathing or coughing. In chronic severe pulmonary tuberculosis, respiratory function declines, often leading to progressive dyspnea or even hypoxia and cyanosis. Complications such as pneumothorax or large pleural effusions can exacerbate dyspnea.
Signs
Early small lesions or those deep in lung tissue often show no abnormal signs. Larger lesions may cause weakened respiratory movement on the affected side, dullness on percussion, and diminished breath sounds or bronchovesicular sounds on auscultation. Since pulmonary tuberculosis commonly affects the apical and posterior segments of the upper lobes and the dorsal segments of the lower lobes, slight dullness may be noted above the clavicles or between the scapulae, and occasional moist rales after coughing may aid diagnosis. Extensive fibrosis or pleural thickening may cause retraction of the affected hemithorax, narrowed intercostal spaces, tracheal deviation, and dullness, with compensatory emphysema on the opposite side.
Tuberculosis infection and the development of pulmonary tuberculosis
Pulmonary tuberculosis is classified into primary and secondary types. Primary pulmonary tuberculosis refers to the initial infection with tuberculosis bacilli in the lungs, commonly seen in children. At this stage, the body's reactivity is low, and local inflammatory responses are mild, with bacilli often spreading to lymph nodes via lymphatic vessels. Secondary pulmonary tuberculosis usually occurs in adults with prior tuberculosis exposure, where the body exhibits some immunity and hypersensitivity. Dormant bacteria in the lungs reactivate, typically forming lesions near the lung apices, rarely spreading to lymph nodes or causing hematogenous dissemination. However, local inflammatory reactions are intense, often leading to caseous necrosis and cavity formation. This differs significantly from primary tuberculosis and may be regarded as a Koch phenomenon occurring within the human body.
The evolution process from infection with subcutaneous node bacteria to the formation of pulmonary subcutaneous nodes (Figure 1), as well as the resulting common clinical types, are described below. It must be noted that most lesions can be absorbed and dissipated or become indurated and calcified at a certain stage of disease progression, especially after the rational use of anti-subcutaneous node chemotherapy drugs, leading to clinical recovery. Only a small number of patients experience worsening disease progression due to excessively low resistance or improper treatment.
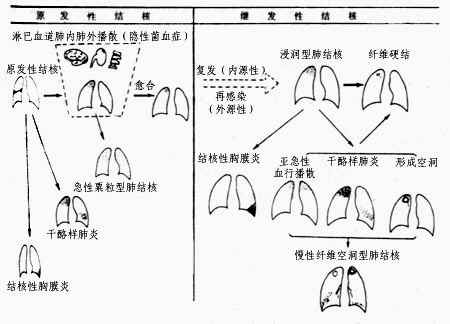
Figure 1 Schematic diagram of the natural course of pulmonary subcutaneous node disease
I. Primary pulmonary subcutaneous node
When the body's resistance is reduced, inhaled subcutaneous node bacteria form exudative lesions in the lungs, typically located at the base of the upper lobe, the middle lobe, or the upper part of the lower lobe (areas with greater lung ventilation). This leads to lymphadenitis and lymphangitis, with both the primary lesion and lymph nodes potentially undergoing caseous necrosis. The primary lung lesion, lymphangitis, and local lymphadenitis are collectively referred to as the primary complex (Figure 2). Primary pulmonary subcutaneous node mostly occurs in children but can also be seen in adults from remote mountainous or rural areas who are newly exposed to urban environments. Most patients may be asymptomatic or exhibit only mild symptoms resembling the common cold, such as low-grade fever, mild cough, decreased appetite, and weight loss, which resolve within weeks. X-rays may reveal the primary lung lesion, lymphangitis, and hilar lymphadenopathy. Most lesions resolve spontaneously or calcify. If the primary lung lesion is near the pleural membrane, it may cause pleuritis when the body is in a hypersensitive state. The primary lung lesion usually resolves quickly, often leaving no trace or only minor calcification. Hilar lymphadenitis may occasionally persist and spread to adjacent mediastinal lymph nodes. Enlarged hilar lymph nodes compressing the bronchi can lead to atelectasis, distal lung inflammation, or secondary bronchiectasis. Hilar or mediastinal lymph node subcutaneous node is more common than the primary complex.
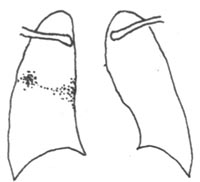
Figure 2 Primary pulmonary subcutaneous node—primary complex
In primary subcutaneous node, the primary lung lesion, especially the subcutaneous node bacteria in the hilar lymph nodes, often enters the bloodstream in small amounts and disseminates to various organs. However, due to the body's strong resistance, the lesions are usually confined to the lung apex (or upper lung), bones, brain, liver, or genitourinary organs, where they gradually heal. However, the subcutaneous node bacteria within these lesions can survive long-term, posing a risk of recurrence (forming secondary subcutaneous node lesions).
II. Hematogenous disseminated pulmonary subcutaneous node
This is one of the more severe forms of pulmonary subcutaneous node. It often develops from primary pulmonary subcutaneous node, but in adults, it is mostly caused by the rupture of extrapulmonary subcutaneous node lesions (e.g., caseous lesions in the genitourinary organs) into blood vessels.
Acute miliary pulmonary subcutaneous node is part of acute systemic hematogenous disseminated subcutaneous node disease. It has an abrupt onset with systemic toxic symptoms, often accompanied by subcutaneous node meningitis. X-rays show dense miliary shadows, approximately 2 mm in diameter, uniformly distributed over a dense reticular background in both lungs, with roughly equal size and density (Figure 3). Early chest X-rays may show no obvious miliary shadows or only diffuse reticular changes, which can be misdiagnosed as cold-damage disease, sepsis, or other febrile illnesses.
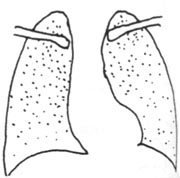
Figure 3 Acute miliary pulmonary subcutaneous node
If the body's resistance is stronger, small amounts of subcutaneous node bacteria enter the lungs in batches via the bloodstream. The hematogenous disseminated lesions are often uneven in size and vary in age, symmetrically distributed in the upper and middle parts of both lungs. This is termed subacute or chronic hematogenous disseminated pulmonary subcutaneous node. The disease progresses slowly, usually without significant toxic symptoms, and patients may be asymptomatic, only discovered incidentally on X-ray. These seasonal lesions are often stable or have already healed with fibrosis.
III. Infiltrative pulmonary subcutaneous node
This is the most common type of pulmonary subcutaneous node. Its symptoms, signs, and X-ray findings can vary greatly depending on the nature, extent, and stage of the lesions.
Primary infection spreads hematogenously (occult bacteremia), and most of the subcutaneous node bacteria that become latent in the lungs gradually die. Only when the body's immunity declines do the latent subcutaneous node bacteria in the lesions have the opportunity to proliferate, forming lesions primarily characterized by exudation and cellular infiltration, accompanied by varying degrees of caseous necrosis. This is known as infiltrative pulmonary subcutaneous node (endogenous infection) (Figure 4). The primary lesion may also directly progress into infiltrative pulmonary subcutaneous node.
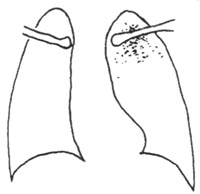
Figure 4 Infiltrative Pulmonary Tuberculosis Subcutaneous Node
In addition, close contact with patients excreting tuberculosis bacilli and repeated respiratory infections can also lead to infiltrative pulmonary tuberculosis subcutaneous node due to reinfection (exogenous infection), but this is relatively rare and does not cause bacteremia. Infiltrative pulmonary tuberculosis subcutaneous node mostly occurs in adult patients, with a slow onset. In the early stages or when the lesion is small, there are often no obvious symptoms or signs. It is frequently discovered during health check-ups or chest X-ray examinations for other reasons. The clinical symptoms depend on the extent of the lesion and the body's reactivity. The lesion is usually located above or below the clavicle, and the X-ray shows patchy or flocculent shadows with blurred edges. When the body is in a hypersensitive state and a large number of tuberculosis bacilli enter the lungs, the lesion undergoes caseous necrosis and liquefaction, leading to cavity formation and bronchial dissemination of the lesion. When infiltrative pulmonary tuberculosis subcutaneous node is accompanied by large areas of caseous necrosis, it often progresses acutely, presenting with severe toxic symptoms, clinically referred to as caseous (or tuberculous) pneumonia. After partial resolution of the caseous necrotic focus, a fibrous capsule forms around it; or if the draining bronchus of the cavity is blocked, the caseous material inside the cavity cannot be expelled and condenses into a spherical lesion, known as a "tuberculoma."
When the lesion is in the stage of inflammatory exudation, cellular infiltration, or even caseous necrosis, appropriate anti-tuberculosis chemotherapy can lead to the absorption and resolution of the inflammation, leaving behind small caseous foci surrounded by fibrous tissue. These gradually dehydrate and dry out, or even calcify, becoming residual nodular lesions, referred to as fibrotic indurated foci or clinical cure. Effective chemotherapy can cause cavities to gradually shrink and close, or even if the tissue defect of the cavity remains, the tuberculosis bacilli within it may be completely eradicated, a condition termed "open healing of the cavity."
IV. Chronic Fibrocavitary Pulmonary Tuberculosis Subcutaneous Node
If pulmonary tuberculosis subcutaneous node is not detected or treated in a timely manner, cavities may persist for a long time, with thickened cavity walls and extensive fibrosis in the lesion. Fluctuations in the body's immunity lead to alternating phases of lesion absorption and repair or worsening and progression, resulting in chronic fibrocavitary pulmonary tuberculosis subcutaneous node. The lesions often undergo repeated bronchial dissemination, with alternating phases of absorption and repair or worsening and progression, leading to chronic fibrocavitary pulmonary tuberculosis subcutaneous node. The lesions frequently exhibit repeated bronchial dissemination, a protracted course, fluctuating symptoms, and the presence of tuberculosis bacilli in the sputum, making it an important source of tuberculosis transmission. X-rays show unilateral or bilateral single or multiple thick-walled cavities (Figure 5), often accompanied by bronchial disseminated lesions and significant pleural thickening. Due to fibrous contraction of the lung tissue, the hilum is pulled upward, and the lung markings appear as willow-like shadows, with the mediastinum pulled toward the affected side. Adjacent or contralateral lung tissue often shows compensatory emphysema, and complications such as chronic bronchitis, bronchiectasis, secondary infections, or chronic cor pulmonale are common. Extensive destruction of lung tissue and proliferation of fibrous tissue can further lead to lobar or whole-lung contraction ("destroyed lung"). These changes can be regarded as sequelae of secondary pulmonary tuberculosis subcutaneous node.
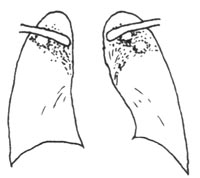
Figure 5 Chronic Fibrocavitary Pulmonary Tuberculosis Subcutaneous Node
In summary, the clinical progression of pulmonary tuberculosis subcutaneous node reflects the outcome of the interaction between the human body and tuberculosis bacilli. When the body's resistance is strong and proper treatment is administered, the lesions may resolve and absorb, or harden and calcify, leading to recovery. Conversely, if the body's resistance is low and appropriate treatment is not provided, the lesions may undergo caseous necrosis, liquefaction, and cavity formation, leading to worsening and progression. If the condition fluctuates, with alternating phases of worsening and repair, old and new lesions may coexist, further complicating into emphysema and cor pulmonale.
bubble_chart Auxiliary Examination
1. Subcutaneous node bacillus examination
is the most specific method for diagnosing pulmonary subcutaneous node. Finding subcutaneous node bacilli in sputum is the primary basis for diagnosing pulmonary subcutaneous node. Acid-fast staining smear microscopy is rapid and simple. Since atypical mycobacteria are still rare in our country, a positive acid-fast bacilli result essentially confirms the diagnosis of pulmonary subcutaneous node. Direct thick smear has a higher positive rate than thin smear and is now widely used. Fluorescence microscopy is suitable for rapid examination of large numbers of specimens. For patients without sputum or children who cannot cough, morning gastric lavage fluid can be used to search for subcutaneous node bacilli. Adults can also undergo fiberoptic bronchoscopy or have their lavage fluid examined for subcutaneous node bacilli. Positive sputum bacilli indicate that the lesion is open and pestilential. If the amount of bacilli excreted is high (more than 100,000 per milliliter), direct smears are likely to be positive, making the patient a source of social pestilence. For lower bacilli counts (less than 10,000 per milliliter), concentration methods can be used.
Culture methods are more precise. In addition to determining the growth and reproductive capacity of subcutaneous node bacilli, they can also be used for drug sensitivity testing and strain identification. Subcutaneous node bacilli grow slowly, and using improved Löwenstein-Jensen medium, it typically takes 4-8 weeks to report results. Although cultures are time-consuming, they are accurate, reliable, and highly specific. They are particularly important when smears are negative or the diagnosis is in doubt. Further drug sensitivity testing of cultured strains can provide references for treatment, especially in retreatment cases.
The specimen can be subjected to polymerase chain reaction (PCR) in vitro to amplify the trace amounts of subcutaneous node bacillus DNA present, which is then detected via electrophoresis. One subcutaneous node bacillus contains about 1 fg of DNA, and as few as 40 bacilli can yield a positive result. This method does not require in vitro pre-culture, is highly specific, and can produce a report in just two days. It is rapid, simple, and can identify bacterial types. However, it may yield false positives or false negatives.
2. Imaging examination
Chest X-ray can reveal the location, extent, presence of cavities, cavity size, and wall thickness of pulmonary lesions. X-ray penetration varies for different types of subcutaneous node lesions, allowing for a rough estimation of the pathological nature of subcutaneous node lesions. It also enables early detection of pulmonary subcutaneous node, assessment of disease progression and treatment efficacy, and aids in determining treatment plans. It must be noted that pulmonary lesions caused by different disease causes may present similar X-ray images, so a diagnosis of pulmonary subcutaneous node cannot be made solely based on X-ray findings.
Combining X-ray films with fluoroscopy can improve diagnostic accuracy, helping to detect small lesions obscured by ribs, mediastinum, diaphragm, or the heart, and allowing observation of the dynamics of the heart, lungs, and diaphragm.
Common X-ray manifestations of pulmonary subcutaneous node include: fibrocalcified indurated lesions, appearing as high-density, sharply marginated spots, streaks, or nodules; infiltrative sexually transmitted disease lesions, appearing as faint, poorly defined cloud-like shadows; caseous lesions, appearing as high-density, unevenly shaded areas with ring-like boundaries and translucent zones (cavities). Pulmonary subcutaneous node lesions are typically located in the upper lungs, unilaterally or bilaterally, persist for a long time, and often show mixed lesions of different natures along with signs of intrapulmonary dissemination.
On chest X-rays, exudative or exudative-proliferative sexually transmitted disease lesions, caseous pneumonia, caseous lesions, and cavities (except for sterilized cavities) all suggest active sexually transmitted disease changes. Proliferative sexually transmitted disease changes, tightly encapsulated caseous indurated lesions, and fibrocalcified lesions are considered inactive sexually transmitted disease changes. Active lesions may still yield subcutaneous node bacilli in sputum. Since pulmonary subcutaneous node lesions are often mixed, they should still be considered active until they fully proliferate or fibrocalcify.
Chest CT scans are helpful for detecting tiny or hidden sexually transmitted disease lesions, assessing lesion extent, and differentiating pulmonary diseases.
3. Subcutaneous node bacillus (commonly referred to as tuberculin) test
is a reference indicator for diagnosing subcutaneous node infection.
Old tuberculin (OT) is a metabolic product of subcutaneous node bacteria, refined from liquid-cultured subcutaneous node bacteria, primarily containing subcutaneous node protein. OT antigens are impure and may cause non-specific reactions. During population screenings, 0.1 ml (5 IU) of a 1:2000 OT dilution can be injected intradermally on the flexor side of the left forearm. After 48–72 hours, the diameter of the skin induration is measured: less than 5 mm is negative, 5–9 mm is weakly positive (indicating subcutaneous node bacterial or subcutaneous node mycobacterial infection), 10–19 mm is a positive reaction, and 20 mm or more or the presence of local blisters and necrosis indicates a strongly positive reaction.
The purified protein derivative (PPD) of tuberculin is refined from the protein of subcutaneous node filtrate, serving as pure tuberculin without causing nonspecific reactions. The internationally commonly used PPD—RT23 has replaced OT. In China, PPD-C derived from human-type subcutaneous node bacteria and BCG-PPD made from the Bacillus Calmette-Guérin (BCG) vaccine both exhibit high purity and are widely used in clinical diagnosis. An intradermal injection of 0.1ml (5IU) with an average induration diameter ≥5mm is considered a positive reaction. Besides local skin reactions, the tuberculin test may occasionally cause systemic reactions. Clinically, 5IU is typically used for diagnosis. If no reaction occurs, another 5IU dose may be administered after one week (due to the tuberculin boosting effect). If the result remains negative, subcutaneous node infection can generally be ruled out.
The tuberculin test remains one of the common methods in the comprehensive diagnosis of subcutaneous node disease, aiding in determining whether subcutaneous node bacterial infection exists. A strongly positive reaction often indicates active subcutaneous node disease. A positive tuberculin test only signifies prior subcutaneous node infection, not necessarily current illness. In China, over 60% of urban adults have been infected with subcutaneous node bacteria, so a general positive result from a 5IU tuberculin test holds little significance. The diagnostic value of the tuberculin test is higher for infants and young children than for adults, as the natural infection rate decreases with younger age. A strongly positive reaction in children under three years old should be regarded as indicative of recent active subcutaneous node infection, warranting treatment. If within two years, the tuberculin reaction changes from <10mm增加至10mm以上,并增加6mm以上時,可認為有新感染。
A negative tuberculin test not only suggests the absence of subcutaneous node bacterial infection but also requires consideration of the following scenarios. After subcutaneous node bacterial infection, it takes 4–8 weeks to establish a sufficient hypersensitivity reaction; before this reaction develops, the tuberculin test may yield a negative result. The use of immunosuppressive drugs like glucocorticoids, malnutrition, or conditions such as measles and whooping cough can temporarily suppress the tuberculin reaction. Severe subcutaneous node disease and critically ill patients may show no reaction or only a weak positive, linked to temporary suppression of immunity and hypersensitivity, which may revert to positive upon recovery. Other conditions, such as lymphocyte immune system defects (e.g., leukemia, lymphoma, sarcoidosis, Acquired Immune Deficiency Syndrome) or advanced age and frailty, often result in negative tuberculin reactions.
IV. Other Examinations
Patients with subcutaneous node disease typically show no changes in blood tests, though severe cases may exhibit secondary anemia. In acute foxtail millet-type pulmonary subcutaneous node, total white blood cell counts may decrease or display a leukemoid reaction. An elevated erythrocyte sedimentation rate (ESR) is common in active pulmonary subcutaneous node but lacks diagnostic specificity; a normal ESR does not exclude active pulmonary subcutaneous node. For patients without sputum or with negative sputum cultures requiring differentiation from other diseases, enzyme-linked immunosorbent assay (ELISA) can detect specific antibodies in serum, potentially aiding in the diagnosis of extrapulmonary subcutaneous node. Bronchoscopy is valuable for detecting endobronchial membrane subcutaneous node, assessing tumors, collecting secretions, relieving obstructions, examining pathogens and exfoliated cells, and obtaining biopsy samples for pathological examination. Superficial lymph node biopsy assists in the differential diagnosis of subcutaneous node.
In recent years, molecular biology and genetic engineering techniques have enabled non-culture methods to detect and identify subcutaneous node bacteria in clinical specimens, demonstrating advantages such as high sensitivity, speed, and specificity. Examples include nucleic acid probes (DNA probes) and chromosomal nucleic acid fingerprinting.
Sputum subcutaneous node bacteria examination is not only the main basis for diagnosing pulmonary subcutaneous node, but also an important indicator for evaluating treatment efficacy and monitoring disease progression. Patients with pulmonary subcutaneous node may exhibit intermittent bacterial excretion in sputum, so multiple consecutive sputum tests should be performed. X-ray examination is an essential method for diagnosing pulmonary subcutaneous node, playing a crucial role in early diagnosis, determining the location, extent, and nature of lesions, understanding their evolution, and selecting treatment options.
In clinical diagnosis, the classification system currently used in China consists of four parts: type of pulmonary subcutaneous node, extent of lesions and location of cavities, sputum bacteria examination, and activity and outcome.
I. Pulmonary subcutaneous node is divided into five types:
Type I: Primary pulmonary subcutaneous node; Type II: Hematogenous disseminated pulmonary subcutaneous node; Type III: Infiltrative pulmonary subcutaneous node; Type IV: Chronic fibrocavitary pulmonary subcutaneous node; Type V: subcutaneous node pleurisy.
II. Extent of lesions and location of cavities
The right and left lungs are divided into upper, middle, and lower lung fields. Lesions in the right lung are recorded above the horizontal line, and lesions in the left lung are recorded below the horizontal line. If there are no lesions in the right lung, it is marked as "(-)". The lungs are divided into upper, middle, and lower fields based on the lower edges of the 2nd and 4th anterior ribs. Cavities are marked with a "0" in the corresponding lung field.
III. Sputum subcutaneous node bacteria examination
Positive or negative sputum bacteria are marked as (+) or (-), with "涂" (smear), "集" (concentration), or "培" (culture) indicating the method used. If the patient has no sputum or the sputum was not examined, it is noted as "no sputum" or "not examined."
IV. Activity and outcome
To determine the activity and outcome of pulmonary subcutaneous node, the patient's clinical manifestations, lung lesions, cavities, and sputum bacteria should be comprehensively assessed. The activity of pulmonary subcutaneous node lesions can be divided into late stage [third stage]:
(1) Progressive stage
Must meet one of the following criteria: newly discovered active sexually transmitted disease lesions; worsening or increase in lesions; new cavity formation or enlargement of existing cavities; positive sputum bacteria.
(2) Improvement stage
Improvement is indicated by one of the following: lesion absorption compared to previous; cavity closure or reduction; sputum bacteria turning negative.
(3) Stable stage
Lesions show no active changes, cavities are closed, and sputum bacteria remain negative (with at least monthly sputum tests) for over 6 months. If cavities persist, sputum bacteria must remain negative for over 1 year.
Open pulmonary subcutaneous node refers to patients in the progressive stage or partial improvement stage, whose sputum apoplexy involving meridians often contains subcutaneous node bacteria, posing a high risk of pestilence, and thus requiring isolation treatment.
Active pulmonary subcutaneous node refers to exudative infiltrative lesions or degenerative sexually transmitted disease lesions such as caseous necrosis, cavity formation, bronchial dissemination, and hematogenous disseminated foxtail millet-type subcutaneous node, with prominent clinical symptoms. Both the progressive and improvement stages are considered active pulmonary subcutaneous node, with almost all progressive stage patients (except for a few, such as acute hematogenous disseminated foxtail millet-type subcutaneous node) excreting bacteria. Some improvement stage patients also continue to excrete bacteria and are classified as open pulmonary subcutaneous node. Others in the improvement stage with negative sputum bacteria are not considered open. All active pulmonary subcutaneous node patients with bacterial excretion in sputum require isolation treatment.
Stable stage patients are classified as inactive pulmonary subcutaneous node and considered preliminarily clinically cured; if after two years of observation, lesions remain stable and sputum bacteria stay negative, they can be deemed clinically cured. If cavities persist, observation for over three years with no changes is required before considering clinical cure.
Example diagnosis: Infiltrative pulmonary subcutaneous node=upper 0 middle/middle smear (+) progressive stage.
bubble_chart Treatment Measures
Anti-subcutaneous node chemotherapy plays a decisive role in controlling subcutaneous node disease. Rational chemotherapy can eliminate bacteria within the lesions, ultimately achieving a cure. Rest and nutritional therapy only serve as auxiliary measures.
I. Anti-subcutaneous node chemotherapy (referred to as chemotherapy)
(1) Principles of Chemotherapy
The main role of chemotherapy is to shorten the pestilence period and reduce mortality, infection rates, and morbidity. For each individual patient, it serves as the primary measure to achieve clinical and biological cure. Rational chemotherapy adheres to the principles of early, combined, appropriate dosage, regular, and full-course use of sensitive drugs for active subcutaneous node disease. "Early" primarily refers to early treatment—administering medication immediately upon detection and diagnosis. "Combined" means using two or more drugs based on the condition and the characteristics of anti-subcutaneous node drugs to enhance and ensure efficacy. "Appropriate dosage" involves prescribing different doses according to varying conditions and individual needs. "Regular" means patients must strictly follow the chemotherapy regimen's prescribed method, adhering to treatment consistently without arbitrarily altering the plan or discontinuing medication without reason, nor intermittently skipping doses. "Full-course" requires patients to complete the entire treatment duration as specified in the regimen, with short-course chemotherapy typically lasting 6–9 months. Generally, initial treatment following these standardized principles achieves a success rate of up to 98%, with a recurrence rate below 2%.
Active pulmonary subcutaneous node is an indication for chemotherapy. Long-standing indurated lesions do not require chemotherapy. For partially indurated lesions with negative sputum bacteria, observation may be appropriate. If X-ray shows no active lesions, sputum bacteria remain negative, and there are no significant subcutaneous node toxic symptoms, chemotherapy is also unnecessary.
1. Early, Combined, Appropriate Dosage, Regular, and Full-Course Medication Active sexually transmitted disease lesions in the exudative stage, with caseous necrosis or even cavity formation, primarily contain A-group subcutaneous node bacteria with vigorous growth and metabolism. Anti-subcutaneous node drugs can exert their maximum bactericidal or bacteriostatic effects. The lesion site's rich blood supply and adequate drug concentration help absorb inflammatory components, shrink or close cavities, and convert sputum bacteria to negative. Thus, early rational chemotherapy for active sexually transmitted disease lesions yields satisfactory results.
Experiments show that each 1g of caseous lesion or cavitary tissue in the lungs contains approximately 10^6–10^10 subcutaneous node bacteria. Subcutaneous node bacteria never exposed to anti-subcutaneous node drugs do not exhibit uniform sensitivity. Roughly 1 in 10^5–10^6 subcutaneous node bacteria may develop resistance to isoniazid or streptomycin due to gene mutation. Simultaneous resistance to both drugs occurs in only 1 in 10^11 bacteria, while resistance to three drugs is even rarer. Thus, monotherapy may eliminate most sensitive bacteria but risks leaving a few resistant strains to proliferate, eventually leading to dominant resistant growth. Combining two or more drugs reduces resistant bacteria and improves efficacy over monotherapy.
Dosage must be appropriate. Insufficient doses fail to achieve effective tissue concentrations and may lead to secondary drug resistance. Excessive doses increase the risk of adverse reactions. Subcutaneous node bacteria grow slowly and occasionally multiply (B, C groups), so maintaining long-term effective drug concentrations in the body is crucial. Regular, full-course medication without premature discontinuation is key to successful chemotherapy.
2. Drugs and Subcutaneous Node Bacteria For a drug to exhibit bactericidal effects in the blood (including within macrophages), its concentration at conventional doses must exceed 10 times the minimum inhibitory concentration (MIC) in vitro. Otherwise, it only has a bacteriostatic effect. At standard doses, isoniazid and rifampin can achieve this level both inside and outside cells, making them full bactericidal agents. Streptomycin and pyrazinamide are also bactericidal agents, but streptomycin works best in an alkaline environment and rarely penetrates phagocytic cells, rendering it ineffective against intracellular subcutaneous node bacteria. Although pyrazinamide can enter phagocytic cells, it only exerts bactericidal effects in an acidic environment, so both are considered semi-bactericidal agents. Ethambutol and sodium para-aminosalicylate are bacteriostatic agents, as their concentrations at conventional doses do not exceed 10 times the MIC. Increasing the dose often leads to adverse reactions.
In the early lesions, most of the subcutaneous node bacteria are extracellular, at which time the bactericidal effect of isoniazid is the strongest, followed by streptomycin. Inflammation lowers the local tissue pH, slowing bacterial metabolism (Group C bacteria), along with some subcutaneous node bacteria phagocytosed within cells (Group B bacteria), making them sensitive to rifampin and pyrazinamide. Eliminating these residual bacteria (Group B) helps reduce future relapses.
(II) Chemotherapy Methods
1. "Standard" Chemotherapy vs. Short-Course Chemotherapy In the past, conventional treatment involved a 12–18 month regimen, termed "standard" chemotherapy. However, due to the lengthy duration, many patients could not complete it, limiting its efficacy. After the introduction of rifampin, combined with other drugs, it was found that a 6–9 month regimen (short-course chemotherapy) achieved the same results as standard chemotherapy. Thus, short-course chemotherapy is now widely adopted. However, this regimen must include two bactericidal drugs, isoniazid and rifampin, which have strong bactericidal (against Group A bacteria) and sterilizing (against Groups B and C bacteria) effects.
2. Intermittent Dosing and Two-Phase Treatment Experiments show that after subcutaneous node bacteria are exposed to drugs for several hours, their growth is often delayed for days. Therefore, regular dosing three times a week (intermittent dosing) can achieve the same effect as daily dosing. During the initial 1–3 months of chemotherapy, drugs are administered daily (intensive phase), followed by intermittent dosing three times a week (consolidation phase). This approach yields results similar to daily dosing, facilitates treatment supervision, and ensures completion of the full course. When using intermittent therapy with three doses per week, combination therapy should still be employed. The doses of isoniazid, rifampin, and ethambutol can be appropriately increased per administration. However, streptomycin, sodium aminosalicylate, and ethionamide, which have more adverse effects, should not have their doses increased per administration (Table 1).
Table 1: Common Anti-subcutaneous node Drugs, Adult Doses, and Major Adverse Reactions
| Drug Name | Abbreviation | Daily Dose (g) | Intermittent Therapy Single Dose (g) | Bacteriostatic Mechanism | Major Adverse Reactions |
| Isoniazid | H, INH | 0.3 | 0.6–0.8 | DNA Synthesis | Peripheral neuritis, occasional liver function impairment |
| Rifampin | R, RFP | 0.45–0.6 * | 0.6–0.9 | mRNA Synthesis | Liver function impairment, allergic reactions |
| Streptomycin | S, SM | 0.75–1.0 △ | 0.75–1.0 | Protein Synthesis | Hearing impairment, vertigo, kidney function impairment |
| Pyrazinamide | Z, PZA | 1.5–2.0 | 2–3 | Pyrazinoic Acid Bacteriostasis | Gastrointestinal discomfort, liver function impairment, hyperuricemia, arthralgia |
| Ethambutol | E, EMB | 0.75~1.0** | 1.5~2.0 | RNA synthesis | Optic neuritis |
| Sodium Aminosalicylate | P, PAS | 8~12*** | 10~12 | Intermediate metabolism | Gastrointestinal discomfort, allergic reactions, liver function impairment |
| Prothionamide | 1321Th | 0.5~0.75 | 0.5~1.0 | Protein synthesis | Gastrointestinal discomfort, liver function impairment |
| Kanamycin | K, KM | 0.75~1.0△ | 0.75~1.0 | Protein synthesis | Hearing impairment, vertigo, renal function impairment |
| Capreomycin | Cp, CPM | 0.75~1.0△ | 0.75~1.0 | Protein synthesis | Hearing impairment, vertigo, renal function impairment |
Note: * Body weight<50kg用0.45,≧50kg用0.6;S、Z、Th用量亦按體重調節;* *前2個月25mg/kg,其後減至15mg/kg;* * *每日分2次服用(其它藥均為每日一次);△老年人每次0.75g。
3. Supervised medication: Anti-subcutaneous node medication should be taken for at least six months, occasionally up to a year and a half, which patients often find difficult to adhere to. It is particularly necessary for medical staff to supervise medication on time, strengthen follow-up visits, and obtain patient cooperation. Administering the medication once daily during the intensive phase can achieve peak blood concentration, which is more effective than multiple daily doses. It also facilitates patient adherence and increases the rate of completing the full course.
(3) Anti-subcutaneous node drugs
The ideal anti-subcutaneous node drugs have bactericidal, sterilizing, or strong bacteriostatic effects, low toxicity, reduced adverse reactions, are inexpensive, easy to use, and have ample supply. After oral administration or injection, the drug should reach effective concentrations in the blood and penetrate phagocytes, the peritoneal cavity, or cerebrospinal fluid, providing rapid and lasting efficacy.
1. Isoniazid (H) has the advantages of strong bactericidal activity, oral administration, few adverse reactions, and low cost. Its main mechanism of action is to inhibit the synthesis of subcutaneous node bacterial deoxyribonucleic acid (DNA) and impede bacterial cell wall synthesis. After oral administration, it is rapidly absorbed, penetrates tissues, crosses the blood-brain barrier, and kills metabolically active or dormant subcutaneous node bacteria both inside and outside cells. The drug concentration in pleural fluid, caseous lesions, and cerebrospinal fluid is also quite high. The usual dose is 300 mg daily (or 4–8 mg/kg per day) for adults, taken orally once daily; for children, the dose is 5–10 mg/kg per day (not exceeding 300 mg daily). In cases of subcutaneous node meningitis and acute foxtail millet-type subcutaneous node, the dose may be appropriately increased (higher doses may cause peripheral neuritis, which can be prevented with 300 mg of vitamin B6 daily; however, high doses of vitamin B6 may also reduce the efficacy of isoniazid, so vitamin B6 is unnecessary when using standard doses of isoniazid). The dose can be returned to the conventional level after acute toxic symptoms subside. Isoniazid is inactivated in the body through acetylation, and the rate of acetylation varies among individuals. Rapid acetylators have lower blood drug concentrations, and it is suggested that intermittent dosing may require an increased dose.
Adverse reactions are rare at conventional doses of this drug, but occasional peripheral neuritis, central nervous system toxicity (excitation or inhibition), and liver damage (elevated serum alanine aminotransferase) may occur. When isoniazid is used alone for 3 months, the drug resistance rate of sputum bacteria can reach 70%.
2. Rifampin (R) is a semi-synthetic derivative of rifamycin and a broad-spectrum antibiotic. Its mechanism of killing subcutaneous node bacteria lies in inhibiting the RNA polymerase of the bacteria, thereby obstructing their mRNA synthesis. Rifampin is effective against both intracellular and extracellular metabolically active and occasionally replicating subcutaneous node bacteria (groups A, B, and C) and is often used in combination with isoniazid. The adult dose is 450–600 mg taken orally once daily on an empty stomach. Adverse reactions to this drug are mild, including occasional transient liver damage in addition to gastrointestinal discomfort and flu-like syndrome. Long-acting rifamycin derivatives such as rifapentine (DL473) have a long half-life in the human body and can be taken orally once a week, with efficacy comparable to daily rifampin. Spiropiperidyl rifamycin (ansamycin, LM427, rifabutin) is more effective than rifampin against certain strains (e.g., Mycobacterium avium complex) that have developed resistance to other anti-subcutaneous node drugs.
3. Streptomycin (S) is a broad-spectrum aminoglycoside antibiotic with bactericidal effects against subcutaneous node bacteria. It interferes with the enzymatic activity of subcutaneous node bacteria, obstructing protein synthesis. Its effect on intracellular subcutaneous node bacteria is limited. Dose: Adults receive 1 g via intramuscular injection daily (those over 50 or with reduced renal function may take 0.5–0.75 g). Intermittent therapy involves 1 g via intramuscular injection twice weekly. Use with caution in pregnant women.
The main adverse reactions of streptomycin involve damage to the eighth cranial nerve, manifesting as vertigo, tinnitus, and deafness. Severe cases should discontinue use immediately, and it is not recommended for those with severe renal impairment. Other allergic reactions include rashes, exfoliative dermatitis, and drug fever, with anaphylactic shock being rare. Monotherapy easily leads to drug resistance. Other aminoglycoside antibiotics, such as kanamycin, capreomycin, and viomycin, also have anti-subcutaneous node effects but are less effective than streptomycin, with similar adverse reactions.
4. Pyrazinamide (Z) can kill subcutaneous node bacteria within phagocytes and in acidic environments. Dose: 1.5 g daily, divided into three oral doses. Adverse reactions include occasional hyperuricemia, arthralgia, gastrointestinal discomfort, and liver damage.
5. Ethambutol (E) has bacteriostatic effects against subcutaneou






