| disease | Congenital Subaortic Stenosis |
In cases of subvalvular aortic stenosis, the aortic valve is mostly normal and tricuspid. Some cases may exhibit slightly thickened valve leaflets or grade I insufficiency. A few patients may also have bicuspid aortic valve stenosis. The left ventricular myocardium shows significant concentric hypertrophy. Subendocardial ischemia can lead to myocardial fibrosis. Occasionally, the degree of hypertrophy in the ventricular septum is more pronounced than in the posterior wall of the left ventricle, making it easily confused with obstructive hypertrophic cardiomyopathy. Approximately one-third of cases of subvalvular fibrous aortic stenosis are accompanied by other congenital cardiovascular anomalies, commonly including ventricular septal defect, interrupted aortic arch, patent ductus arteriosus, tetralogy of Fallot, atrial septal defect, pulmonary valve stenosis, or right ventricular outflow tract stenosis.
bubble_chart Clinical Manifestations
Subvalvular aortic stenosis accounts for approximately 25% of congenital aortic stenosis, with two common types:
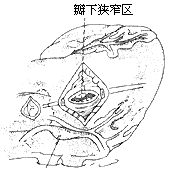
(1) Fibrous membrane type: A thin, annular fibrous membrane is located about 1 cm below the aortic valve ring, partially or completely encircling the left ventricular outflow tract. Blood flow must pass through a small central or eccentrically located opening in the membrane to enter the aorta, causing obstruction. In a few cases, fibrous adhesions may exist between the membrane and the aortic valve leaflets or between the membrane and the anterior mitral leaflet.
(2) Fibrous tunnel-type subaortic stenosis: This type is less common, accounting for about 20% of subvalvular aortic stenosis. The fibrous tissue forms a tubular structure extending from 1–2.5 cm below the aortic valve ring into the distal segment of the left ventricular outflow tract. The fibrous tunnel generally has an internal diameter of about 1 cm and a length ranging from 1 cm to 3 cm. Longer tunnels are often associated with a narrow aortic valve ring and severe flow obstruction (Figure 5).
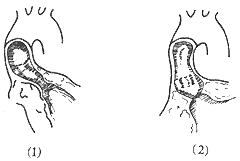
**Figure 5 Types of Subvalvular Aortic Stenosis**
(1) Fibrous tunnel-type stenosis (2) Fibrous membrane-type stenosis
Clinical manifestations of subvalvular fibrous aortic stenosis: X-ray, electrocardiogram, and cardiac catheterization findings are similar to those of valvular aortic stenosis, but an early systolic click is rarely heard. In cases where the mobility of the anterior mitral leaflet is restricted by fibrous stenosis, a diastolic murmur due to mitral regurgitation may be heard at the apex. Chest X-rays typically do not show post-stenotic dilation of the ascending aorta, and there are no signs of calcification in the aortic valve leaflets. In a few cases, continuous pressure recordings from the left ventricular outflow tract and aorta during cardiac catheterization may reveal a third pressure curve intermediate between the left ventricle and aorta, with systolic pressure equal to the aorta and diastolic pressure equal to the left ventricle.
Selective left ventricular angiography can demonstrate either a short, localized annular membrane-type stenosis or a longer tunnel-type stenosis in the left ventricular outflow tract.
Two-dimensional echocardiography: In the long-axis view of the left ventricle, the fibrous membrane located about 1 cm below the aortic valve ring and its central small opening can be directly visualized. Alternatively, a longer fibrous tubular stenosis may be seen in the left ventricular outflow tract, with the posterior wall of the stenotic segment corresponding to the anterior mitral leaflet.
bubble_chart Treatment Measures
Subvalvular aortic stenosis does not produce grade III left ventricular outflow obstruction in infants and young children, and the clinical symptoms are not severe, so surgical treatment is rarely required during infancy. However, as childhood progresses, the obstructive lesion develops more rapidly. Due to the impact of turbulent blood flow post-stenosis, the aortic valve leaflets often thicken, leading to aortic valve insufficiency and an increased risk of endocarditis.
**Surgical Treatment:** In 1956, Brock reported closed transventricular dilatation of the stenosis. In 1960, Spencer began performing direct resection of the stenotic lesion under cardiopulmonary bypass. Rostan and Konez in 1974 and Konno in 1975 independently applied aortico-ventriculoplasty to treat fibrous tunnel-type subvalvular aortic stenosis.**Surgical Procedure:**
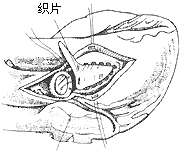
**Resection of Subaortic Fibrous Membrane:** Cardiopulmonary bypass is combined with hypothermia, cold cardioplegia, and local cardiac cooling. A transverse incision is made in the ascending aorta to identify the lesion's anatomical relationship with the anterior mitral leaflet and the ventricular septum. The base of the left coronary cusp and the adjacent non-coronary cusp connect to the anterior mitral leaflet, while the right coronary cusp is close to the ventricular septum. The junction of the right and non-coronary cusps corresponds to the membranous ventricular septum and the atrioventricular bundle. The aortic valve leaflets are retracted to expose the subaortic fibrous membrane, which is typically 1–2 mm thick. The membrane is lifted with forceps and incised from the ventricular septum using a scalpel. Care must be taken near the anterior mitral leaflet to avoid perforating the membranous ventricular septum. The membrane's attachment to the anterior mitral leaflet should be fully excised to free the leaflet and ensure unrestricted movement. When excising the membrane tissue below the right coronary cusp and the muscular ventricular septum, excessive depth should be avoided to prevent injury to the conduction system. If the membrane adheres to the aortic valve leaflets, it should be carefully dissected and removed. After complete resection, the aortic incision is closed in two layers, with the last 1–2 sutures left untied until the left heart is filled with blood to expel residual air (Figure 1). The aortic clamp is then removed. Cardiac activity resumes, and cardiopulmonary bypass is discontinued once the body temperature rises above 35°C.
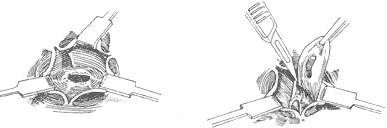
(1) Exposure (2) Resection of the fibrous membrane
**Figure 1** Resection of subaortic fibrous membrane
For cases with **grade III aortic insufficiency**, aortic valve replacement should be performed concurrently after resection of the subaortic stenosis.
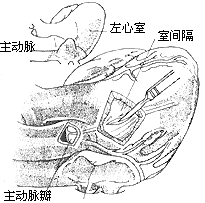
Fibrous tunnel-type stenosis resection: In this type of subaortic stenosis, the aortic valve annulus is often small. To relieve left ventricular outflow tract obstruction, most cases require concurrent aortic valve replacement. During aortico-ventricular plasty, extracorporeal circulation combined with cardiac cold cardioplegia and local myocardial cooling is used to protect the myocardium. After establishing extracorporeal circulation, the ascending aorta is clamped. The fatty tissue on the anterior wall of the ascending aortic root is dissected to identify the position of the right coronary artery orifice. A longitudinal incision is made on the anterior wall of the ascending aortic root, with the right edge of the incision approximately 7 mm from the right coronary artery to ensure sufficient aortic wall tissue for suturing the aortic incision without compromising right coronary artery blood flow. The lower edge of the incision extends downward and leftward, cutting through the aortic valve annulus at the junction of the right and left coronary cusps and extending into the anterior wall of the right ventricular outflow tract below the pulmonary valve, thereby exposing both sides of the ventricular septum. From the lower edge of the aortic valve annulus incision, a longitudinal incision is made through the thickened ventricular septum at the supraventricular crest, completely opening the subaortic tubular stenosis. The aortic valve is excised, and a sufficiently large prosthetic aortic valve is implanted, with most (about 60%) of the prosthetic valve's sewing ring sutured and fixed to the aortic valve annulus. A spindle-shaped Dacron patch is trimmed to match the shape, size, and length of the ventricular septal incision and aortic incision. The lower end of the patch is sutured and fixed to the left side of the ventricular septum, with the sutures on the right ventricular side reinforced with small Dacron pledgets. The patch is placed on the left side of the ventricular septum; due to the higher pressure in the left ventricular cavity, the patch is pressed tightly against the ventricular septum, reducing the likelihood of left-to-right shunting in the septal repair area. The middle portion of the patch is sutured and fixed to the sewing ring of the prosthetic valve, completing the prosthetic valve replacement. The upper portion of the patch is continuously sutured to the edges of the ascending aortic incision, while the right ventricular outflow tract incision is continuously sutured with pericardium or a patch. The upper half of the pericardial patch is sutured and covers the surface of the Dacron patch already used to repair the ascending aortic incision (Figure 2), completing the intracardiac procedure. After aortico-septal plasty, the aortic valve annulus diameter can increase by 5–8 mm, while the left ventricular outflow tract can also expand by 50%.
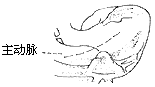
(1) Incision
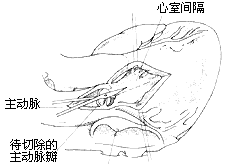
(2) Longitudinal incision of the thickened ventricular septum and subvalvular tubular stenosis of the main stirred pulse
(3) Resection of the main stirred pulse valve, replacement with an artificial valve membrane, and placement of a suitably sized patch. The lower end of the patch is sutured and fixed to the left side of the ventricular septum, with the sutures on the right ventricular side reinforced by small polyester pads. The middle part of the patch is sutured and fixed to the sewing ring of the artificial valve membrane
(4) The right ventricular outflow tract incision is closed with pericardial sutures
Figure 2: Resection of Fibrous Tunnel-Type Stenosis
For cases of subvalvular fibrous tunnel-type stenosis of the main stirred pulse where the main stirred pulse valve annulus and leaflets are normal and do not require replacement of the main stirred pulse valve membrane, a transverse incision can be made at the root of the ascending main stirred pulse and another about 2 cm below the pulmonary stirred pulse valve in the right ventricular outflow tract. A right-angle clamp is inserted into the left ventricular outflow tract through the main stirred pulse incision, and the clamp can be palpated below the ventricular septum through the right ventricular incision. Under the conduction exercise of the right-angle clamp, the ventricular septum is incised from the right ventricular side. The septal incision is parallel to the left ventricular outflow tract, approximately 2–3 cm long, with the upper edge not extending beyond the main stirred pulse valve. The subvalvular fibrous tunnel of the main stirred pulse is dissected and resected. A polyester patch is used to repair the ventricular septal incision and enlarge the left ventricular outflow tract. The main stirred pulse and right ventricular incisions are then closed (Figure 3). Some surgeons advocate performing a bypass procedure outside the heart, connecting a larger-caliber artificial vessel with a biological valve membrane between the left ventricle and the ascending main stirred pulse, descending thoracic main stirred pulse, or abdominal main stirred pulse. One end of the artificial vessel is anastomosed end-to-end to the incision at the left ventricular apex, while the end with the artificial valve membrane is anastomosed end-to-side to the main stirred pulse (Figure 4).
(1) A transverse incision is made at the root of the main stirred pulse and another about 2 cm below the pulmonary stirred pulse valve in the right ventricular outflow tract
(2) Resection of the subvalvular fibrous tunnel of the main stirred pulse
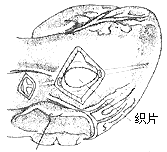
(3) Repair of the ventricular septal incision with a polyester patch

(4) Closure of the main stirred pulse and right ventricular incisions
Figure 3: Resection of Subvalvular Fibrous Tunnel-Type Stenosis of the Main Stirred Pulse (No Valve Membrane Replacement Required)
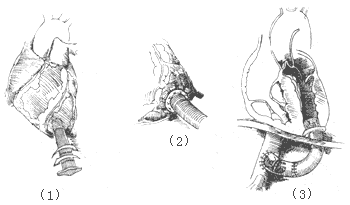
Figure 4: Anastomosis of an Artificial Vessel with an Artificial Valve Membrane
Therapeutic effect: Congenital subvalvular aortic stenosis rarely requires surgical intervention during infancy, resulting in a lower operative mortality rate (approximately 5%) compared to valvular stenosis. The mortality rate for aortoventricular plasty is relatively higher, nearing 10%, with a notably elevated incidence of postoperative conduction bundle injuries. Some case series report conduction block occurrence rates as high as 50%. Postoperatively, the systolic pressure gradient between the left ventricle and aorta significantly decreases, with cardiac function improving to Class I in about 80% of patients. However, during a 15-year follow-up, approximately 40% of cases progressed to advanced-stage mortality. Causes of advanced-stage mortality include residual left ventricular outflow tract obstruction, recurrent stenosis, atrioventricular block, and aortic or mitral valve insufficiency.




