| disease | Premature Beat |
| alias | Premature Contraction, Extrasystole, Premature Beat, Premature Beat |
Premature beat, also known as premature contraction or extrasystole, is an early ectopic heartbeat. It can be classified into four types based on the site of origin: sinus, atrial, atrioventricular junctional, and ventricular. Among these, ventricular premature beats are the most common, followed by atrial, while sinus premature beats are rare. Premature beats are a common type of ectopic rhythm. They can occur in the context of sinus rhythm or other ectopic rhythms (such as atrial fibrillation). They may occur occasionally or frequently, and can be irregular or appear regularly after every or several normal beats, forming bigeminy or coupled premature beats.
bubble_chart Etiology
Premature beats can occur in normal individuals. However, patients with cardiac neurosis Guanneng or organic heart disease are more prone to them. Emotional agitation, nervous tension, fatigue, indigestion, excessive smoking, alcohol consumption, or drinking strong tea can all trigger episodes, or they may occur without obvious triggers. The toxic effects of substances such as Rehmannia, barium agents, quinidine, sympathomimetic drugs, chloroform, cyclopropane anesthetics, potassium deficiency, as well as cardiac surgery or catheterization can also induce them. Conditions such as coronary heart disease, advanced stage mitral valve disease, heart disease, myocarditis, hyperthyroid heart disease, and mitral valve prolapse are often associated with a higher incidence of premature beats.
It can be generated through various means.
(1) Abnormal impulse formation due to abnormal automaticity: ① Under certain conditions, such as when a sinus impulse reaches an ectopic pacemaker site, the threshold potential decreases and the slope of diastolic depolarization changes due to the Wedensky phenomenon, resulting in premature beats (Figure 1). ② Pathological changes in the membrane permeability of atrial, ventricular, or Purkinje fiber cells to different ions cause fast-response fibers to transform into slow-response fibers, accelerating diastolic automatic depolarization and enhancing automaticity, thereby generating premature beats.
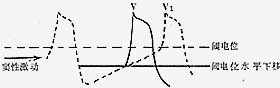
Figure 1: Schematic of the Wedensky Phenomenon
A sinus impulse reaches the ectopic pacemaker, lowering the threshold potential level at that site. As a result, the diastolic depolarization of the ectopic pacemaker reaches the threshold potential before the basic rhythm pacemaker, causing a premature beat.
(2) Reentry phenomenon—circus movement reentry or focal microreentry: If the reentry pathway is the same, the morphology of the premature beat remains consistent; if the conduction velocity during reentry is consistent, the coupling interval between the premature beat and the preceding beat is fixed.
(3) Parasystole
(4) Triggered activity
bubble_chart Clinical Manifestations
Premature beats may be asymptomatic or may cause palpitations or a sensation of a skipped heartbeat. Frequent premature beats can lead to symptoms such as lack of strength and dizziness (due to reduced cardiac output). In individuals with preexisting heart disease, this may trigger or worsen colicky pain or heart failure. Auscultation may reveal irregular heart rhythms, with a longer compensatory pause following a premature beat. The first heart sound of a premature beat is often enhanced, while the second heart sound is usually weakened or absent. When premature beats occur in bigeminy or trigeminy, a long pause can be heard after every two or three heartbeats. If a premature beat is interpolated between two regular beats, it may present as three consecutive heartbeats. Pulse palpation may reveal an intermittent absence of the pulse.
bubble_chart Auxiliary Examination
[ECG manifestations]
The common electrocardiographic feature of premature beats is the appearance of one or more P-QRS complexes that occur earlier than the basic rhythm.
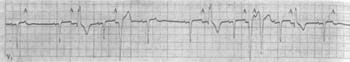
Figure 2 Frequent atrial premature beats with aberrant ventricular conduction
Frequent atrial premature beats (marked A) with premature deformed P′ waves on the T wave of the preceding cardiac cycle. The conducted QRS complex differs from the sinus rhythm due to aberrant ventricular conduction. The first and eighth marked A waves are not followed by QRS complexes, indicating blocked atrial premature beats.
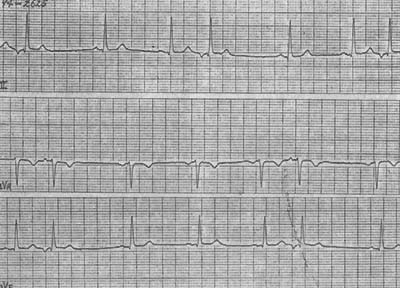
Figure 3 Atrioventricular junctional premature beats
(3) Ventricular premature beats The QRS complex appears prematurely, with an abnormal morphology, and the duration is mostly >0.12s. The T wave is in the opposite direction to the main QRS wave, and the ST segment shifts with the T wave. There is no P wave before the QRS complex. Ventricular premature beats originating near the bundle branches may not show a widened QRS complex. Most ventricular premature beats are followed by a complete compensatory pause. When the basic rhythm is slow, ventricular premature beats may occur between two sinus beats, forming interpolated ventricular premature beats. Occasionally, ventricular premature beats may conduct retrogradely to the atria, producing retrograde P′ waves, often seen on the ST segment of the ventricular premature beat.
Atrial and ventricular premature beats can be classified into two types based on their relationship with the basic rhythm, using ventricular premature beats as an example:
1. Coupled type All premature beats have a fixed interval with the preceding QRS complex, and this type is more common (Figure 4).
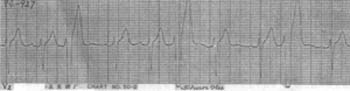
Figure 4 Coupled ventricular premature beats presenting as trigeminy
2. Parasystolic type The premature beats are not coupled with the preceding QRS complexes, but there is a fixed relationship between the premature beats themselves. The longest interval between premature beats is an integer multiple of the shortest interval, and ventricular fusion beats often occur (Figure 5).
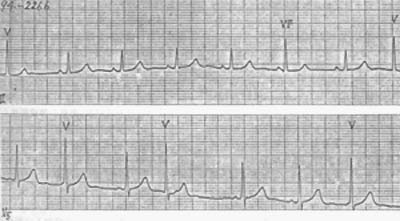
Figure 5 Parasystolic ventricular premature beats
Premature beats have no fixed coupling interval with the preceding cardiac cycle. Late-occurring premature beats may coincide with sinus impulses, forming ventricular fusion beats (the 6th beat in lead II).
Experimental studies have found that the above-mentioned pattern can be altered due to sinus or ectopic impulses, which undergo slow decremental conduction in the protective entrance block zone, generating subthreshold potentials distal to the block. These potentials influence the spontaneous depolarization of abnormal parallel rhythm impulse formation, causing it to occur earlier, be delayed, or be completely suppressed. This phenomenon is referred to as electrotonic modulated parasystole.
Atrial or ventricular premature beats are sometimes generated by two or more ectopic pacemakers. The electrocardiogram shows two or more types of premature beats with different morphologies and unequal coupling intervals, known as multifocal premature beats. Consecutive two or three and more premature beats are referred to as couplets and short runs of tachycardia, respectively.
(1) Medical history and symptoms: Due to varying patient sensitivity, there may be no obvious discomfort or only sensations of palpitation, precordial discomfort, or a feeling of skipped heartbeats. Inquiring about a history of hypertension, coronary heart disease, cardiomyopathy, or rheumatic heart disease helps identify the cause of premature beats and guide treatment. Pay attention to recent histories of common cold, fever, or diarrhea to assess whether acute sexually transmitted disease viral myocarditis is present. The use of digitalis drugs, antiarrhythmic medications, and diuretics may sometimes induce premature beats.
(2) Physical examination findings: In addition to the positive signs of the underlying heart disease, auscultation may reveal an early heartbeat within a regular rhythm, followed by a longer pause (compensatory pause). The first heart sound of the premature beat is enhanced, while the second heart sound is weakened, and the corresponding pulse may also be weaker or absent.
(3) Auxiliary examinations: Electrocardiography (ECG) is diagnostic for premature beats. Atrial premature beats show an early QRS complex preceded by an abnormal P wave, followed by an incomplete compensatory pause, with the QRS morphology usually similar to the normal QRS. Junctional premature beats exhibit an early QRS complex identical to the normal QRS, without a preceding P wave, and a complete compensatory pause. Ventricular premature beats present with a broad and abnormally shaped QRS complex and a complete compensatory pause. A 24-hour Holter monitor can record the frequency, patterns, and treatment efficacy of premature beats. For suspected myocarditis, cardiac enzyme tests may be performed. Echocardiography can detect cardiomyopathy and some cases of coronary heart disease. Patients on long-term diuretics or suspected of digitalis toxicity should undergo blood electrolyte testing, and blood digitalis concentration measurement if necessary.
bubble_chart Treatment Measures
The treatment principles should be determined by considering the presence or absence of organic heart disease, whether it affects cardiac output, and the potential for developing severe arrhythmias.
Most premature beats without an underlying organic heart disease do not require special treatment. For symptomatic cases, reassurance is advisable. Premature beats induced by excessive tension, emotional agitation, or exercise may be managed with sedatives and β-blockers.
For frequent episodes, significant symptoms, or cases associated with organic heart disease, it is essential to promptly identify the disease cause and triggers of the premature beats and provide corresponding treatment. At the same time, the potential for fatal outcomes should be accurately recognized, with active treatment of the disease cause and symptomatic management.
In addition to disease cause treatment, antiarrhythmic drugs may be selected. Atrial and atrioventricular junctional premature beats are mostly treated with class Ia, Ic, II, or IV drugs acting on the atria and atrioventricular junction, while ventricular premature beats are often managed with class I and III drugs acting on the ventricles (refer to the drug classification above and also to Part VII, "Introduction to Clinical Pharmacology"). Potentially life-threatening ventricular premature beats often require urgent intravenous medication, with class Ib drugs as the first choice. In the initial stage of acute myocardial infarction, intravenous lidocaine is still commonly the first choice. Post-myocardial infarction, β-blockers are often used if there are no contraindications. For primary or secondary QT prolongation syndrome, class I drugs are contraindicated; primary cases may be treated with β-blockers, phenytoin, or carbamazepine. For secondary cases, the disease cause should be addressed, and treatment with isoproterenol or atrial or ventricular pacing is advisable.
Recent research (CAST 1989) suggests that antiarrhythmic drugs may increase the risk of mortality. Even in patients with heart disease, controlling ventricular premature beats does not provide evidence of reducing sudden death rates (except for post-myocardial infarction β-blocker use). Therefore, the benefits and risks of antiarrhythmic drugs should be carefully weighed. In China, a large multicenter trial with long-term follow-up in non-myocardial infarction arrhythmia patients (mainly premature beats) showed that supraventricular arrhythmias treated with propafenone or moricizine, and ventricular arrhythmias treated with propafenone, moricizine, or mexiletine, had certain efficacy without serious cardiac events. However, close follow-up monitoring of effects and potential adverse reactions is still necessary during treatment, especially in patients with cardiac dysfunction.





