| disease | Inhalation Injury |
Inhalation injury refers to chemical injury to the respiratory tract caused by inhaling toxic fumes or chemicals, and in severe cases, it can directly injure the lung parenchyma. It mostly occurs in large-area burns, especially in patients with head and facial burns.
bubble_chart Pathogenesis
The primary cause of inhalation injury is thermal effects, but simultaneously inhaling large amounts of unburned smoke, carbon particles, and irritating chemicals can also injure the respiratory tract and alveoli. Therefore, inhalation injury is a mixed injury caused by both thermal and chemical factors.
Inhalation injury is related to the injurious environment. It often occurs in poorly ventilated or confined spaces, especially during explosive combustion. In such environments, the concentration of flames is high, temperatures are elevated, and dispersal is slow, making it difficult for victims to escape immediately. Additionally, in confined spaces, incomplete combustion produces large amounts of carbon monoxide and other toxic gases, leading to poisoning and unconsciousness, or even fatal asphyxiation. During explosive combustion, high-temperature, high-pressure, high-velocity airflow and dense toxic gases can cause injury to the deep respiratory tract and lung parenchyma. Furthermore, victims standing or running while shouting may inhale hot flames, which is another cause of injury.
The mechanisms of inhalation injury include the following aspects:
1. Direct thermal injury to the respiratory tract
Thermal effects include dry heat and damp-heat. Flames and hot air are classified as dry heat, while hot steam is damp-heat. When hot air is inhaled, the vocal cords reflexively close. Additionally, dry heat has poor heat conduction, and the upper respiratory tract has a heat-exchange function that absorbs significant heat to cool the air. By the time dry heat reaches the carina at the bronchial bifurcation, the temperature may drop to 1/5–1/10 of its original level. Thus, dry heat often causes upper respiratory tract injury. Damp-heat air has a heat capacity about 2000 times greater than dry heat and a conductivity about 4000 times higher, with slower heat dissipation. Therefore, damp-heat can injure not only the upper respiratory tract and trachea but also the bronchi and lung parenchyma.
2. Harmful substances causing respiratory tract injuryInhaled smoke contains not only particles but also large amounts of harmful substances, including carbon monoxide, nitrogen dioxide, sulfur dioxide, nitrogen peroxide, hydrochloric acid, hydrocyanic acid, aldehydes, ketones, and others. These substances can directly injure the respiratory tract through thermal effects. Toxic gases can irritate the throat and cause bronchospasm, as well as chemically injure the respiratory tract. Water-soluble substances such as ammonia, chlorine, and sulfur dioxide combine with water to form acids or alkalis, leading to chemical burns. Nitrogen compounds react with water and salts on the respiratory mucosa, generating nitric acid and nitrites. The former directly corrodes the respiratory tract, while the latter, after absorption, binds with hemoglobin to form methemoglobin, causing tissue hypoxia. Hydrocyanic acid inhibits cytochrome oxidase, disrupting oxygen transfer and suppressing cellular respiration. Aldehydes reduce ciliary activity, impair alveolar macrophage function, and damage capillaries, leading to pulmonary edema. Smoke from burning polyurethane contains about 50 ppm of acrolein. Inhaling air with just 5.5 ppm of acrolein can cause chemical respiratory tract injury and pulmonary edema, while 10 ppm can be fatal within minutes. The toxicity of hydrogen cyanide and carbon monoxide is additive. At temperatures reaching 1000°C, polyurethane foam decomposes, producing large amounts of hydrogen cyanide. A serum cyanide concentration of 100 μmol/L can be lethal.
Inhaled carbon monoxide in smoke leads to carbon monoxide poisoning, which can be fatal in severe cases. Inhalation of air containing 5% carbon monoxide can cause poisoning. Its toxic effects include:
(1) Carbon monoxide binds with hemoglobin to form carboxyhemoglobin. The dissociation of carboxyhemoglobin is 1/3600th the rate of oxyhemoglobin dissociation, and carbon monoxide’s affinity for hemoglobin is 200–300 times greater than that of oxygen. This impairs blood oxygen transport, causing systemic tissue hypoxia.(2) It reduces the ability of cellular enzyme systems to utilize oxygen. Carbon monoxide competes with oxygen for cytochrome oxidase receptors, directly inhibiting cellular respiration.
(3) Carbon monoxide binds with myoglobin, reducing oxygen delivery to tissues.
In addition, during a fire, high concentrations of carbon dioxide are also produced. Carbon dioxide can exacerbate the symptoms of carbon monoxide poisoning and worsen tissue hypoxia.
bubble_chart Pathological Changes
Pathological and physiological changes of inhalation injury
Inhalation injury, due to the stimulation of thermal and chemical toxins, causes congestion and edema of the respiratory mucosa, increased secretions, and bronchospasm, which increases airway resistance and leads to ventilation disorders. The inhalation of smoke particles first inactivates pulmonary surfactant. Pulmonary surfactant maintains alveolar surface tension, keeps the alveoli in a certain state of expansion, and prevents alveolar collapse. Niman found that the minimum surface tension of the lungs in dogs significantly increased after smoke inhalation, rising from an average of 6.8 dyn/cm to 22 dyn/cm. In the early stages of inhalation injury, non-obstructive, scattered focal atelectasis without airway blockage can be observed, primarily due to the inactivation of surfactant.
The mortality rate of patients with inhalation injury is relatively high, with most cases complicated by pulmonary edema and acute respiratory failure. Therefore, pulmonary edema is the main pathological change in inhalation injury. Inhalation injury-induced lung injury is generally considered to be mediated by free radicals, primarily initiated by oxygen free radicals. After lung injury, changes in vascular permeability and pulmonary edema occur. Damage to pulmonary microvessels allows large amounts of fluid to enter the pulmonary interstitium, while bronchial veins are also severely damaged. Fluid from these two pathways accumulates in the interstitium, forming interstitial pulmonary edema. When interstitial fluid further increases, hydrostatic pressure rises, or other factors come into play, the alveolar wall barrier can be compromised, allowing interstitial fluid to enter the alveoli and form intra-alveolar edema. After inhalation injury, plasma protein breakdown and extravasation through the vascular wall reduce plasma colloid osmotic pressure, while increased interstitial osmotic pressure can also promote pulmonary edema. The inactivation of pulmonary surfactant, alveolar collapse, and loss of intra-alveolar pressure can further cause fluid to shift into the pulmonary interstitium and alveoli, leading to pulmonary edema and interstitial edema. During resuscitation and anti-shock treatment, fluid may accumulate in the interstitium, exacerbating interstitial edema.
After inhalation injury, tracheal mucosal cells undergo degeneration and necrosis, cilia disappear, and the natural defense barrier is lost. The ability of the airway to expel mucus and clear bacteria and foreign particles is weakened, while the immune function of pulmonary macrophages/monocytes is reduced, making the lungs more susceptible to infection. Due to airway obstruction, pulmonary edema, and lung infections, respiratory difficulties arise, respiratory resistance increases, gas exchange significantly declines, arterial oxygen partial pressure decreases, carbon dioxide partial pressure rises, and ultimately, acute respiratory insufficiency may occur.bubble_chart Clinical Manifestations
In grade III inhalation injury, different clinical and pathological changes manifest as the condition progresses, leading to its division into three stages.
1. Respiratory insufficiency stage
In grade III inhalation injury, the first two days post-injury constitute the respiratory insufficiency stage. The primary symptom is dyspnea, which typically persists for 4–5 days before gradually improving or worsening into respiratory failure, leading to death. Dyspnea results from extensive bronchial injury or lung parenchymal injury, causing ventilation and gas exchange impairments, as well as ventilation-perfusion mismatch, leading to progressive hypoxemia, with blood PaC2 <7.8 kPa. Lung auscultation may reveal dry and wet rales as well as wheezing.
2. Pulmonary edema stage
Pulmonary edema can occur as early as within one hour post-injury, but most cases develop within four days. Clinically, obvious symptoms of pulmonary edema are present. This is mainly due to increased pulmonary capillary permeability, airway obstruction, and ventilation impairment, resulting in tissue hypoxia. At this stage, there is no left heart failure. Inappropriate early treatment or excessive fluid infusion can further predispose to pulmonary edema.
3. Infection stage
Between 3–14 days post-injury, the condition enters the infection stage. Damage to the tracheal and bronchial mucosal cilia impairs the mechanical clearance of foreign bodies in the airways. Concurrently, local and systemic immune function declines, and the injured lungs become more susceptible to bacterial infection. Necrosis and shedding of the airway mucosa may form ulcers, which persist and become foci of pulmonary infection. Pulmonary infections often follow mechanical obstruction and atelectasis. Severe infections can trigger systemic infections.
Clinical Classification of Inhalation Injury
There is currently no unified standard for the classification of inhalation injury. Some categorize it based on severity into mild, moderate, and severe, or simply mild and severe; others classify it by the site of injury, such as upper airway, lower airway, and lung parenchymal injury. Currently, the three-grade classification system is widely adopted domestically (Figure 1).
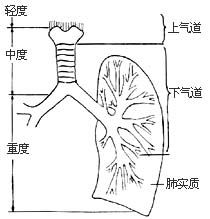
1. Grade I Inhalation Injury
Refers to injury above the glottis, including the nose, pharynx, and glottis. Clinical manifestations include nasopharyngeal pain, cough, increased salivation, and difficulty swallowing; local mucosal congestion, swelling, or blister formation, or mucosal erosion and necrosis. Patients do not exhibit hoarseness or dyspnea, and lung auscultation shows no abnormalities.
2. Grade II Inhalation Injury
Refers to injury above the carina, including the larynx and trachea. Clinical manifestations include irritative cough, hoarseness, dyspnea, carbon particles and sloughed tracheal mucosa in sputum, and laryngeal edema leading to airway obstruction, with inspiratory stridor. Lung auscultation reveals weakened or coarse breath sounds, occasionally accompanied by wheezing and dry rales. Patients often develop tracheitis and aspiration pneumonia.
3. Grade III Inhalation Injury
Refers to injury below the bronchi, including the bronchioles and lung parenchyma. Clinical manifestations include severe dyspnea immediately or within hours after injury, unrelieved by tracheostomy; progressive hypoxia, cyanosis, tachycardia, agitation, delirium, or unconsciousness; productive cough with early-onset pulmonary edema, expectoration of bloody frothy sputum; sloughing of necrotic mucosa may lead to atelectasis or asphyxia. Lung auscultation reveals diminished or coarse breath sounds, wheezing, followed by dry and wet rales. Patients with severe lung parenchymal injury may die within hours due to extensive alveolar damage and severe bronchospasm causing acute respiratory failure.
Diagnosis of Inhalation Injury
The diagnosis of inhalation injury is primarily based on the circumstances of injury and clinical manifestations, combined with laboratory tests, X-rays, and special examinations to confirm the presence, location, and severity of inhalation injury.
1. Medical History
A detailed inquiry into the circumstances of injury is necessary. A history of burns in confined spaces or exposure to irritative or corrosive gases should raise suspicion of inhalation injury.
2. Clinical Manifestations
Patients may present with burns on the face, head, and neck, particularly around the mouth and nose, singed nasal hair, mucosal congestion and edema in the oral cavity and pharynx, and blister formation; cough with carbon particles in sputum; dyspnea, hypoxia, dysphoria; hoarseness, sloughing of tracheal mucosa; expectoration of bloody frothy sputum in pulmonary edema, with diminished or coarse breath sounds, or dry and wet rales on lung auscultation. In inhalation injury, laryngotracheal edema narrows the airway, leading to dyspnea, high-pitched breath sounds, and sometimes a sharp whistling sound, necessitating tracheostomy. Grade III inhalation injury presents with progressive dyspnea early on. However, even without inhalation injury, extensive burns may lead to acute respiratory insufficiency and dyspnea, which should be noted.
3. X-ray Examination
It was previously believed that X-rays had no diagnostic significance for inhalation injuries. However, Wang Tianyi et al. (1980) and Yang Zhiyi et al. (1982) concluded through animal experiments and clinical observations that taking X-ray films in the right anterior oblique position revealed significant tracheal stenosis 2 to 6 hours after injury, with spotted shadows visible inside the trachea, reduced translucency, and irregular mucous membranes. Early signs of tracheal stenosis could serve as indicative X-ray changes for inhalation injuries. In cases of pulmonary edema, diffuse, ground-glass shadows, interlobar effusions, hilar enlargement, linear or crescent-shaped shadows may appear. Pulmonary infections may present as central infiltrates or diffuse, dense infiltrates. Occasionally, balloon-like hyperlucency due to compensatory lung hyperinflation, as well as pneumothorax shadows caused by alveolar rupture or emphysematous bulla rupture, may be observed.
4. Special Examinations
(1) Fiberoptic Bronchoscopy: Fiberoptic bronchoscopy allows direct visualization of the injury extent to the pharynx, vocal cords, trachea, and bronchial mucosa, as well as the identification of injury sites. Since it enables sampling, drainage, and lavage within the airway, it also serves as a therapeutic tool. Dynamic observation via fiberoptic bronchoscopy can reveal the progression and outcome of pathological changes.
Findings in Inhalation Injury: In upper airway inhalation injury, abnormalities such as pharyngeal edema, congestion, blister formation, ulceration, or hemorrhage are observed. The glottis is usually visible, but in grade III injuries, severe mucosal edema may obscure the glottis, with the pear-shaped sinus disappearing and the ventricular folds approximating. In lower airway inhalation injury, findings include mucosal congestion and edema, prominent vascular networks, significant luminal narrowing, indistinct or exposed cartilage rings, and mucosal sloughing leading to ulceration and hemorrhage. Bronchial openings may appear red and swollen or closed, obstructed by sloughed mucosa or secretions. Foreign materials such as smoke particles, secretions, blood, necrotic mucosa, or purulent exudate may also be present. Additionally, functional dysregulation of the trachea and bronchi may be observed: normally, the trachea and bronchi widen in diameter during inspiration and narrow during expiration, but post-injury, expiration may lead to luminal narrowing or closure, with delayed or absent cough reflex.
Fiberoptic bronchoscopy can be performed via oral or nasal insertion, or directly through a tracheostomy if present. However, bronchospasm due to stimulation during the procedure may cause hypoxia. Examination is not feasible for injuries beyond the tertiary bronchi or alveolar units. There is also a risk of introducing exogenous infections.
(2)133Xenon Lung Scintigraphy with Continuous Scintiphotography: Moylan first applied this method in 1972 for diagnosing inhalation injury, considering it a safe and reliable early diagnostic tool with only a 13% discrepancy compared to autopsy results. This examination is typically conducted within 48 hours post-injury. The radioactive isotope 133Xenon (22×107–74×107 Bq or 6×10-3–20×10-3 Ci) is administered intravenously in saline, with scintiphotography performed every 15 seconds until 133Xenon is completely cleared. Normally, 133Xenon clears from the lungs within 90–150 seconds, termed a normal scan; delayed clearance beyond 150 seconds indicates an abnormal scan. Segmental retention or incomplete clearance signifies inhalation injury, with focal areas of increased radioactive density visible on scintiphotography.
False positives may occur in patients with pre-existing chronic obstructive pulmonary diseases like bronchitis or bronchiectasis. Hyperventilation yields a false-negative rate of about 5%. By day 14 post-injury, approximately 80% of initially abnormal scans normalize, rendering this test unreliable for early diagnosis beyond day 3. While its accuracy reaches 87%, it only confirms the presence and location of inhalation injury, not its severity.
(3) Exfoliative Cytology Scoring: Ambiavagar first reported in 1974 the use of bronchial secretion analysis to diagnose inhalation injury by examining cellular morphology, structural changes, and the presence of smoke particles. Post-injury, ciliated cells exhibit morphological alterations such as ciliary loss, disappearance of terminal plates, waxy basophilic cytoplasm, and pyknotic nuclei, with severe cases showing rupture or dissolution.
Method: Under local anesthesia, specimens are obtained via direct laryngoscopy or tracheostomy. 2ml of saline is instilled into the trachea, and tracheal secretions are aspirated after 30 seconds. The secretions are immediately smeared, fixed, and stained with Papanicolaou. The score is calculated based on the morphology and structural changes of ciliated cells. One point is given for intact cilia, one point for the presence of a terminal plate, one point for pale blue cytoplasm, and one point each for nuclear subcutaneous nodes and nuclear size, totaling a maximum score (Figure 11-8). Each smear is evaluated for 200 ciliated cells, with a total possible score of 1200. Normal adults score 1044±89, children score 754±158, while severe inhalation injury cases score only 12–208, mild cases score 276–446, and burn patients without inhalation injury score 1068–1140.
5. Pulmonary function tests
(1) Blood gas analysis: After inhalation injury, PaO2 decreases to varying degrees, mostly below 8 kPa (60 mmHg). In cases with similar burn areas but without inhalation injury, PaO2 is generally >10.67 kPa (80 mmHg). The PaO2/FIO2 ratio decreases (normal >53.2 kPa), and A-aDO2 rises early, with the degree of increase serving as a prognostic indicator. If PaO2 progressively declines and A-aDO2 increases significantly, it suggests severe disease and poor prognosis.
(2) Pulmonary function testing: This is more sensitive for lower airway inhalation injuries. Key measurements include forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), maximum expiratory flow-volume curve (MEFV), peak flow rate, flow rate at 50% vital capacity, and respiratory mechanics (lung compliance, airway resistance, pulmonary resistance, etc.). After grade III inhalation injury, small airways and lung parenchyma are affected, leading to increased airway resistance. The peak flow rate at 50% vital capacity may drop to 41.6±14.3%, lung compliance decreases, pulmonary resistance significantly increases, MEFV falls markedly below normal, and FEV1 and FVC show abnormalities earlier. These changes result from airway obstruction, so pulmonary function testing has certain significance in predicting disease progression.
bubble_chart Treatment Measures
The treatment options for inhalation injury are relatively limited, as it involves metabolic and internal environment disturbances, pathophysiological changes in pulmonary function, and is often complicated by other injuries. Therefore, the treatment principles still focus on symptomatic management according to the stages of its progression.
1. Maintain airway patency and prevent or relieve obstruction.
(1) Endotracheal intubation and tracheostomy: Inhalation injury can lead to airway obstruction early on due to tissue and mucosal edema, secretion blockage, bronchospasm, etc. Therefore, timely endotracheal intubation or tracheostomy should be performed to relieve obstruction and maintain airway patency. Indications for endotracheal intubation include: ① Grade III facial burns, especially around the mouth and nose, with potential laryngeal obstruction; ② Worsening glottic edema; ③ Difficulty in expelling airway secretions, accompanied by worsening wheezing and hypoxia. Endotracheal intubation should not be prolonged (generally no more than one week), as it may exacerbate laryngeal edema, cause laryngeal ulceration, or even lead to glottic stenosis.
Indications for tracheostomy include:
① Severe supraglottic edema with circumferential eschar on the face and neck;
② Severe bronchial mucus fistula disease;
③ Complicated by ARDS requiring mechanical ventilation;
④ Complicated by severe brain trauma or cerebral edema;
⑤ Endotracheal intubation lasting more than 24 hours. Tracheostomy can immediately relieve obstruction, facilitate drug instillation and tracheal lavage, and allow for bronchoscopy and mechanical ventilation. However, tracheostomy also increases the risk of airway and pulmonary infections, which can be avoided with proper procedures, enhanced postoperative care, and preventive measures.
(2) Escharotomy for decompression: Inhalation injury with circumferential eschar on the neck, chest, or abdomen can compress the airway and blood vessels, restrict thoracic and diaphragmatic movement, impair respiration, exacerbate dyspnea, and reduce cerebral blood supply, leading to cerebral hypoxia. Therefore, timely escharotomy for decompression in these areas is crucial for improving respiratory function and preventing cerebral hypoxia.
(3) Pharmacotherapy: For bronchospasm, aminophylline 0.25g can be administered intravenously slowly every 4–6 hours. Alternatively, salbutamol aerosol spray can be used to dilate the bronchi and relieve spasm. If bronchospasm persists, hormone therapy can be initiated. Hormones also help mitigate symptoms of increased capillary permeability caused by acute inflammation, reduce edema, stabilize alveolar surfactant, and stabilize lysosomal membranes. Since hormones increase the incidence of pulmonary infections, early high-dose intravenous administration is recommended, with dexamethasone being more effective than hydrocortisone. Zhu Peifang et al. reported that early comprehensive treatment with dexamethasone, 654-2, and oxygen in dogs with grade III smoke inhalation injury accelerated CO elimination and improved pulmonary function.
(4) Humidification: Humidification helps prevent damage to the tracheal and bronchial mucosa due to dryness, enhances ciliary activity, prevents secretion crusting, and is critical for avoiding mucus blockage, preventing atelectasis, and reducing pulmonary infections. Nebulized inhalation can be used for airway drug therapy to relieve spasm, reduce edema, prevent infection, and facilitate sputum expulsion. Typically, a solution of 20ml normal saline containing one ampoule each of dexamethasone, gentamicin, and α-chymotrypsin is used for nebulization.
2. Ensure adequate blood volume.
Improving Pulmonary Circulation In the past, it was believed that after inhalation injury, due to increased pulmonary capillary permeability and fluid extravasation, pulmonary edema was likely to occur. Therefore, fluid intake should be restricted during early shock resuscitation to prevent pulmonary edema. This understanding is one-sided because, in cases of inhalation injury accompanied by superficial skin burns, fluid is lost not only from the burned area of the body surface but also from the damaged airways and lungs. Therefore, proper fluid resuscitation should be carried out based on changes in urine output, blood pressure, and vital signs to maintain adequate blood volume. Avoiding fluid restriction is crucial to prevent the inability to maintain effective circulation, which could ultimately lead to poor tissue perfusion and further aggravate tissue damage.
The pulmonary circulation is a low-pressure, low-resistance, high-flow system. Inhalation injury can increase pulmonary vascular resistance, while hypovolemia further reduces pulmonary artery pressure, leading to pulmonary circulatory disorders and even right heart failure. Therefore, cardiotonic drugs such as strophanthin K and cedilanid (lanatoside C) can be used to improve pulmonary circulation. Low-molecular-weight dextran can reduce blood viscosity and decrease red blood cell aggregation, which is beneficial for improving microcirculation.
3. Maintain gas exchange function and correct hypoxemia.
(1) Oxygen therapy: ① Oxygen concentration: Oxygen therapy can be divided into four categories based on concentration—low (24–35%), medium (35–60%), high (60–100%), and hyperbaric oxygen (2–3 atm). The oxygen concentration is calculated as:
Oxygen concentration (%) = 21% + 4 × oxygen flow rate
The goal of oxygen therapy is to increase PaO2 to normal levels. If PaO2 is low while PaCO2 is normal, low or medium concentrations of oxygen can be administered. In cases of hypercapnia or respiratory failure, controlled oxygen therapy should be applied, with the oxygen concentration not exceeding 35%. ② Duration of oxygen administration: Generally, prolonged oxygen administration should not exceed 50–60% concentration for more than one day, and pure oxygen should not be administered for more than 4 hours. Prolonged inhalation of high-concentration oxygen can injure the lungs, causing mild symptoms such as chest pain and cough, or severe complications like decreased lung compliance, aggravated dyspnea, muscle weakness, confusion, and even death. ③ Methods of oxygen delivery: In addition to nasal cannula oxygen delivery, methods include oxygen masks, oxygen tents, and mechanical ventilation. For respiratory insufficiency caused by inhalation injury, nasal cannula or mask oxygen delivery is often ineffective, and positive-pressure oxygen therapy or mechanical ventilation is usually required.
(2) Mechanical ventilation: Patients with inhalation injury often exhibit varying degrees of respiratory insufficiency. If treatment is inadequate, respiratory failure may occur, endangering life. Ventilators are an effective measure for treating respiratory failure. Mechanical ventilation is achieved through ventilators, providing assisted breathing to improve ventilation and gas exchange, maintain effective tidal volume, correct hypoxia, and prevent carbon dioxide retention.
Mechanical ventilation is a symptomatic treatment and emergency measure, and timing its use is crucial. Indications for ventilator use include:
① Clinical manifestations: Difficulty breathing, respiratory rate >35 breaths/min, confusion, dysphoria, unrelieved after tracheostomy, eschar decompression, and oxygen therapy; presence of necrotic tissue shedding in the airway; excessive secretions with inability to cough, etc.
② Blood gas analysis: After high-concentration oxygen administration, PaO2 remains below 7.8 kPa or PaCO2 exceeds 6.5 kPa.
③ Pulmonary signs and X-ray findings: In early respiratory failure, chest X-rays show reduced transparency and increased, thickened lung markings, inconsistent with dyspnea symptoms. When dry or wet rales appear and chest X-rays reveal patchy shadows, the condition is often in an advanced stage.
Although mechanical ventilation can effectively improve respiratory function, it increases the risk of pulmonary infection. Therefore, thorough disinfection of equipment and tubing, adherence to correct operating procedures, prevention of cross-infection, and reduction of pulmonary infection risks are essential.
Currently, commonly used mechanical ventilation includes positive pressure ventilation and high-frequency ventilation. Positive pressure ventilation: Most ventilators used clinically are positive pressure ventilators. During mechanical positive pressure ventilation, gas is delivered into the lungs under positive pressure, increasing the pressure in the thoracic cavity and lungs. Consequently, it may have adverse effects on the circulatory and respiratory systems. Therefore, contraindications must be strictly observed. Conditions where airway pressure may worsen the disease—such as pulmonary bullae, high-pressure pneumothorax, massive hemoptysis, and acute myocardial infarction—should not be treated with this method.
a. Intermittent Positive Pressure Breathing (IPPB): During inhalation, positive pressure is generated to push air into the lungs. During exhalation, the pressure drops to atmospheric level, and gas is expelled through the elastic recoil of the chest wall and lung tissue.
b. End-Inspiratory Positive Pressure Breathing (EIPB): At the end of inhalation and before exhalation, the exhalation valve remains closed for an instant before exhalation begins. This helps expand small airways, increasing effective ventilation.
c. Positive End-Expiratory Pressure Breathing (PEEP): Positive pressure is generated during inhalation to push air into the lungs. During exhalation, the airway pressure remains higher than atmospheric pressure, preventing alveolar collapse due to conditions like effusion or atelectasis. This expands the gas exchange surface and improves blood oxygen levels.
d. Intermittent Mandatory Ventilation (IMV): The mechanical ventilation rate is set at half or one-tenth of the normal respiratory rate. When the ventilator is not delivering breaths, the patient can practice spontaneous breathing. As the condition improves and spontaneous breathing recovers, the ventilator can be gradually withdrawn.
e. Expiratory Retard: A cover with small holes is placed at the expiratory port to increase expiratory resistance and prolong exhalation time, preventing small airway collapse during exhalation.
Intermittent positive pressure breathing is a commonly used method and can also deliver oxygen under positive pressure. If PaO2 remains below 6.7–8 kPa after intermittent positive pressure breathing and high-concentration oxygen therapy, it should be promptly switched to positive end-expiratory pressure breathing. During use, closely monitor the patient’s cardiovascular function, blood gases, blood pressure, and pulse rate, as well as the degree of jugular vein distension, to adjust pressure levels in a timely manner. The PEEP value is generally set between 294–784 Pa and should not exceed 1.5 kPa, as excessive pressure and gas volume can cause varying degrees of complications. High-Frequency Ventilation (HFV): Ventilation with a frequency exceeding 60 breaths per minute is called high-frequency ventilation. It features low airway pressure, low pulmonary artery pressure, and reduced cardiac compression compared to conventional positive-pressure ventilation (HFPPV). Clinically, high-frequency jet ventilation (HFJV) is commonly used (Table 1).
Table 1 Various Methods and Characteristics of High-Frequency Ventilation (HFV)
| Method | Ventilation Frequency | |
| Breaths/Min | Hz | |
| High-Frequency Positive Pressure Ventilation (HFPPV) | 60–100 | 1–1.8 |
| High-Frequency Jet Ventilation (HFJV) | 110–400 | 1.8–6.7 |
| High-Frequency Oscillatory Ventilation (HFO) | 400–2400 | 6.7–40 |
The connection pathways for high-frequency ventilation include the percutaneous endotracheal method, the bronchoscopic method, the oronasal airway method, the nasopharyngeal catheter method, and the most commonly used jet needle connection with an endotracheal tube or tracheostomy tube. Clinically, the frequency range for high-frequency jet ventilation is generally 120–200 breaths per minute.
High-frequency ventilation has its advantages, but also its disadvantages. For example, it has poor ability to overcome airway resistance and is less effective at removing carbon dioxide. However, since carbon dioxide diffuses about 20 times more efficiently than oxygen, early use does not lead to carbon dioxide retention. If used alternately with positive-pressure ventilation, it can compensate for the excessive removal of carbon dioxide caused by hyperventilation. Additionally, high-frequency ventilation still has some issues, such as dampness transformation, alveolar collapse, and changes in lung compliance, so caution should be exercised when using it long-term.
(3) Membrane-type oxygenator (membrane lung): The membrane-type oxygenator (membrane lung, ECMO) consists of multiple parallel collagen membrane units, with a thin layer of blood flowing between the membranes. Oxygen and blood do not come into direct contact. Its therapeutic principle involves oxygenating the patient's blood extracorporeally, temporarily replacing lung function. This avoids lung injury caused by mechanical ventilation and reduces lung load, aiding in the treatment and recovery of diseased lungs.
The membrane-type oxygenator is mainly used for treating acute respiratory failure, with few reports on its use for inhalation injuries. However, based on its mechanism of action, it can also be used to treat respiratory insufficiency caused by inhalation injuries. This facilitates airway and lung lavage as well as drug perfusion, promoting the healing of inhalation injuries.
Prevention and treatment of infection After inhalation injury, due to damage to the airways and lungs, impaired ciliary function, inability to promptly expel airway secretions and foreign bodies, and decreased local and systemic resistance, infections of the airways and lungs often occur. Once infection sets in, if treatment is
deficient, it can lead to complications such as acute respiratory failure and become a significant source of systemic infection, potentially triggering sepsis. Thorough removal of foreign bodies and necrotic mucosal tissue from the airways, ensuring unobstructed drainage, is a fundamental measure for preventing and treating infection. Additionally, strict aseptic techniques and disinfection protocols must be followed to rigorously control cross-infection between wounds, lungs, and wounds. Regular smears and cultures of airway secretions should be performed to select sensitive antibiotics. Furthermore, systemic supportive therapy should be enhanced to improve immune function, which is crucial for infection prevention and treatment.




