| disease | Cytomegalovirus Infection |
CMV infection is distributed worldwide, with humans being the only host of CMV. The infection rates vary among different countries and economic conditions. Adult CMV infection is closely related to immune function.
bubble_chart Epidemiology
CMV infection is distributed worldwide, with humans being the only host of CMV. The infection rates vary among different countries and economic conditions. Adult CMV infection is closely related to immune function. For example, those receiving immunosuppressive therapy due to organ transplantation often develop the disease because of latent viruses in the donated organs or transfused blood, or due to the activation of latent viruses by immunosuppression. The incidence of CMV infection is high in patients with Acquired Immune Deficiency Syndrome.
The source of the pestilence is patients and their acute carriers. The virus can be excreted in milk, saliva, and urine, persisting for several weeks to years. Humans are susceptible to infection, with multiple transmission routes. It is classified as a sexually transmitted disease, often resulting from infection via semen, cervical secretions, tears, and feces (oral-genital contact, analingus). (See Table 1)
Homologous CMV infection poses a serious risk in blood transfusion and organ transplantation. Multiple transfusions or a single large-volume transfusion increases the risk of primary and recurrent infections, with the risk being even higher when leukocyte-containing blood is transfused. The CMV infection rate is also high after organ or bone marrow transplantation.
CMV Infection Routes (Cycle) Table 1
CMV belongs to the herpesvirus group and is the largest virus in the human herpesvirus family, with a maximum size composed of 162 capsomeres forming a regular icosahedron. It has a typical herpesvirus structure. CMV can only proliferate in human fibroblast cell cultures and cannot grow in other animal cells. Its proliferation is very slow, and initial isolation requires more than a month to observe distinctive cellular changes: cells become rounded, swollen, with enlarged nuclei, and a large eosinophilic inclusion body surrounded by a "halo" appears around the nucleus.
CMV survives for a maximum of 2 hours in 20% ether. It can be effectively inactivated at pH <5, after 30 minutes at 56°C, or after 5 minutes of ultraviolet irradiation. The infectivity of CMV is unstable to freeze-thaw cycles or storage at -20°C or -50°C, and 10% household bleach can significantly reduce its infectivity.CMV infection can lead to a reduction in the body's immune function, particularly a decline in cellular immunity. CMV infection significantly impacts the development of the thymus and the functions of splenic cells, mononuclear phagocytes, NK cells, and CTL cells.
I. Effects on the Thymus and Spleen
In laboratory studies of acute CMV infection in newborn guinea pigs, thymic development was suppressed, and T-cell numbers decreased. In adult mice infected with CMV, CMV was detected in 88% of the thymuses.
CMV infection affects spleen function, reducing the proliferation of splenic lymphocytes in response to ConA stimulation and significantly lowering IL-2 production by splenic cells.
II. Effects on Immune Cells
The immune suppression caused by CMV infection is related to viral replication within cells. CMV can replicate in mononuclear phagocytes, T cells, B cells, and some unidentified mononuclear cells, with mononuclear phagocytes being the most susceptible to CMV infection. Lymphocytes play a crucial regulatory and effector role in immune responses. After CMV infection, multiple immune functions of lymphocytes can be impaired.
CMV infection often manifests as acute mononucleosis. Peripheral blood lymphocytes show weakened proliferative responses to mitogens, CMV antigens, and HSV antigens, reduced interferon induction levels, and a decrease in the CD4/CD8 ratio from 1.7±0.7 to 0.2±0.2, along with reduced T-cell activity. These changes can persist for a considerable time; even 10 months after infection, the T-cell subset ratios in most patients have not fully returned to normal.
NK cells have the effect of antagonizing the spread of CMV. NK cells actively participate in the entire process of anti-CMV infection, but high NK activity is not necessarily a protective response; rather, it serves as evidence of active infection. Although NK cells cannot prevent the occurrence of primary CMV infection, once infection is present, NK cells can emerge early in CMV infection, playing a role in limiting its spread and localizing the infection. NK cells and CTL cells are important effector cells against CMV. In the early stages of CMV replication, before the production of sexually transmitted disease viral particles, they can lyse infected cells, preventing the virus from spreading between cells late abortion. In mouse models, during the first 3–5 days of viral activity, the antiviral effect is mediated by NK cells, and NK cell activity can be enhanced by IFN. Between 6–21 days, cytotoxic activity of CTL cells is observed in the spleen and peripheral blood. The level of NK and CTL cell activity determines the host's susceptibility to CMV infection and the ease of recovery. However, during CMV infection, the activity of NK and CTL cells is also severely affected. Additionally, specific cellular immunity plays a role in preventing CMV reinfection. A study examining T-cell responses in 20 kidney transplant recipients with CMV infection found that 14 exhibited CMV cytotoxic responses, while the 6 who lacked such responses experienced severe clinical outcomes. Thus, the presence of specific T cells helps prevent CMV reinfection.
After infection with CMV, the body can produce various antibodies. Although specific antibodies, including neutralizing antibodies, are present in breast milk, cervical secretions, and saliva, CMV can still be detected, indicating that these antibodies cannot prevent viral spread. Antibodies passively acquired by the fetus from the mother cannot block infections transmitted in utero, through the birth canal, or via breast milk. Studies have shown that injecting 0.2 ml of high-titer anti-CMV globulin into the peritoneal cavity or veins of mice before a lethal CMV attack can fully protect the animals from death. When challenged again with CMV one month later, all animals survived, demonstrating that antibodies can reduce the virulence of CMV.
After initial infection, CMV persists indefinitely in host cells in a latent state. It may affect multiple tissues and organs, and autopsies suggest that the lungs, liver, pancreas, salivary glands, central nervous system, and intestines may also serve as sites of viral latency. The severity of congenital infection is associated with the inability to produce precipitating antibodies and the T-cell response to CMV. Following CMV infection in children and adults, activated T lymphocytes with a suppressor-cytotoxic phenotype appear in peripheral blood. If the host's T-cell function is impaired, latent virus may reactivate and cause various syndromes. Chronic stimulation after tissue transplantation provides conditions for CMV reactivation and disease induction. Certain potent immunosuppressants targeting T cells, such as anti-thymocyte globulin, are associated with a high incidence of clinical CMV syndromes. Additionally, CMV can functionally act as a cofactor, reactivating latent HIV infection.
bubble_chart Clinical Manifestations
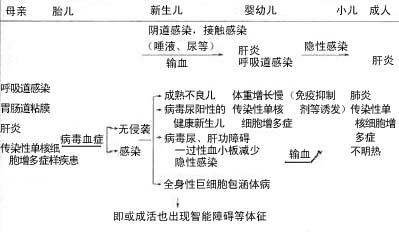
The natural history of CMV infection is complex. Following primary infection, viral shedding often persists for weeks, months, or even years before the infection becomes latent. Recurrent infections with renewed viral shedding are common. Even many years after primary infection, latent virus may reactivate, and reinfection with different antigenically distinct strains can occur. The clinical manifestations of CMV infection are related to the individual's immune function and age. As shown in Table 2, whether through vertical transmission, horizontal spread, or iatrogenic infection, the symptoms and signs are highly diverse.
Table 2 Clinical Course and Types of CMV Infection (Baerlocher et al.)
1. Neonatal infection (congenital) a. Severe type: jaundice, anemia, hepatosplenomegaly, thrombocytopenic purpura, central nervous system involvement, pneumonia, myocarditis) b. Mild type: apparent death, cardiomegaly, hyperbilirubinemia |
2. Postnatal asymptomatic period, reinfection with CMV during infancy (congenital/acquired?) a. Systemic type b. Respiratory type (whooping cough-like) c. Hepatosplenomegaly d. Gastrointestinal type e. Renal type? |
3. Acquired type a. Influenza-like type b. Mononucleosis type c. Respiratory type d. Gastrointestinal type e. Hepatitis f. Due to special medical immunosuppression g. Asymptomatic type? |
4. Consequences of items 1, 2, and 3: mental and motor developmental delays |
Regarding acquired CMV infection, it is clinically common to see post-transfusion mononucleosis, vasculitis, retinitis, pneumonia, and gastrointestinal infections due to immune dysfunction. Moreover, most patients are complicated by Guillain-Barré syndrome.
The diagnosis of CMV infection cannot be made based solely on clinical manifestations. Isolation of the virus from clinical specimens, along with a fourfold or greater increase in antibody levels or persistently elevated antibody titers, will aid in the diagnosis.
I. Virus Isolation
The best specimens for isolation include saliva, urine, genital secretions, breast milk, and white blood cells. These are inoculated into human fibroblast cells for propagation and isolation. Cytopathic effects (CPE) may appear within one day or several weeks. After fixation and HE staining, giant cells can be observed, featuring intranuclear inclusion bodies, perinuclear halos, and eosinophilic cytoplasmic inclusion bodies, resembling "owl's eye." Monoclonal or polyclonal antibody fluorescence staining can also be used for detection.
II. Serum Antibody Detection
The most commonly used methods include complement fixation (CF), indirect immunofluorescence (IIF), enzyme immunoassay (EIA), indirect hemagglutination (IHA), and radioimmunoassay (RIA) to detect CMV-IgG and IgM antibodies. When a single serum sample confirms past CMV infection, additional serum samples should be collected immediately and at intervals of 2, 4, and 8 weeks. The combination of virus isolation and serological testing can diagnose primary infection.
III. DNA Probe
This method has been widely applied for CMV detection, with 32P-labeled probes being the most sensitive. For certain specimens, hybridization methods may be more sensitive than virus isolation.
IV. Polymerase Chain Reaction (PCR)
(1) Specimen Collection and Processing
Specimens include patient blood, urine, and glandular tissue. Buffy coats prepared from whole blood can be stored at -80°C; urine specimens can be stored in liquid nitrogen. Repeated freeze-thaw cycles should be avoided for frozen specimens.
Urine specimens are centrifuged at 2500 rpm for 10 minutes to remove cellular debris, and the supernatant is collected. Since urine contains PCR inhibitors, pretreatment with polyethylene glycol (PEG6000) is required: mix 50μl of urine supernatant with 50μl of 20% PEG6000 and 25μl of 2mol/L NaCl, then incubate in an ice bath for 6 hours. Centrifuge at 15,000 rpm for 30 minutes to collect the precipitate. Further centrifuge at 6400 rpm for 3 minutes; aspirate the supernatant as much as possible and resuspend the precipitate in distilled water. This suspension can be directly used for PCR amplification. Prior to amplification, heat at 100°C for 10 minutes and rapidly cool in an ice bath.
(2) Template DNA Preparation
1. Preparation of Template DNA from Blood Specimens
Method A: Add NaCl to serum to a final concentration of 150mmol/L. Take 10μl of this serum, heat at 70°C for 45 seconds, and then proceed directly to PCR amplification.
Method B: Preparation from Buffy Coat Pellet (BCP): ① Wash BCP twice with PBS and collect the precipitate. ② Add 500μl of 6mol/L guanidine hydrochloride and homogenize. ③ Add 30μl of 10mmol/L EDTA, 10mmol/L NaCl, 30μl of 20% Sarcosyl, and 5μl of proteinase K (10mg/ml), mix well, and react at 60℃ for 1h. ④ Add 1130μl of ethanol and precipitate at -20℃ for 1~2h. ⑤ Centrifuge to collect the DNA precipitate. ⑥ Wash twice with 70% ethanol, and finally suspend the precipitate in a small amount of 10mmol/L Tris-HCl, 1mmol/L EDTA. Store at 4℃ for later use.
2. Preparation of Template DNA from Frozen Tissue Samples: ① Cut a 5–10 μm frozen tissue section using a cryostat and place it in a 1.5 ml plastic tube. ② Add 10% buffered formalin. ③ After 10 minutes, centrifuge for 1–2 minutes, gently decant the supernatant, and wash the pellet twice with ethanol. ④ Dry at room temperature for 10–60 minutes. ⑤ Add extraction buffer (100 mmol/L Tris-HCl, 4 mmol/L EDTA, pH 8.0, 400 μg/ml proteinase K) to just cover the pellet (approximately 50–100 μl); crush the pellet. Formalin-fixed or paraffin-embedded tissues can also be processed similarly after dewaxing and drying. ⑥ Incubate at 37°C overnight. ⑦ Heat in boiling water for 7 minutes to inactivate proteinase K. ⑧ Centrifuge to collect the supernatant, and use 1–10 μl for PCR amplification.
3. Preparation of Template DNA from Urine Samples: ① Mix 100 μl of urine supernatant with 100 μl of 6 mol/L guanidine isothiocyanate, 7 μl of 2 mol/L NaCl, and 20 μl of glass powder suspension (DNA PREP, Asahi Glass Co, Tokyo). ② Incubate at room temperature for 10 minutes, then centrifuge at 6400 rpm for 2 minutes to collect the pellet. ③ Wash the pellet once with 50% ethanol, 10 mmol/L Tris-HCl (pH 7.4), and 50 mmol/L NaCl, then centrifuge at 6400 rpm for 1 minute. ④ Repeat the washing step twice and collect the pellet. ⑤ Add 50 μl of distilled water and incubate at 55°C for 15 minutes. ⑥ Centrifuge at 15000 rpm for 2 minutes and collect the supernatant (containing HCMV DNA) for PCR amplification. Heat the suspension at 100°C for 10 minutes and rapidly cool in an ice bath.
(III) Primers and Probes
The design of primers and probes is based on the published sequences of the promoter region and the first four exons of the CMV immediate-early protein gene, the advanced-stage antigen gp64 gene, and the phosphoprotein pp71 gene. Commonly used primers and probes are listed in Table 3.
(IV) PCR Amplification Steps
1. 10× Reaction Buffer: 100 mmol/L Tris-HCl (pH 8.4), 500 mmol/L KCl, 25 mmol/L MgCl 2 , 2 mg/ml gelatin.
Taq DNA Polymerase: 5 U/μl.
10× dNTP: 2.0 mmol/L each of the four dNTPs.
Primers: 100 pmol/L.
2. Conventional PCR Amplification
10× Reaction Mixture (50 μl): Reaction Buffer: 5 μl
10× dNTP: 5 μl
Template DNA: 5 μl
Taq DNA Polymerase: 0.2 μl (IU)
Primers: 0.5 μl each
Distilled Water: 3.8 μl
Add 1–2 drops of mineral oil.
Heat the reaction mixture at 94°C for 5 minutes; then perform 35–40 cycles of 95°C for 30 s, 55°C for 40 s, and 72°C for 60 s.
Table 3 Primers and Probes Used for CMV PCR Amplification
Primer and Probe | Sequence (5′→3′) | Position | Fragment Size |
IE1 | CCACCCGTGGTGCCAGCTCC | Early Gene | 159 (bp) |
IE2 | CCCGCTCCTCCTGAGCACCC | | |
IE3 (probe) | CTGGTGTCACCCCCAGAGTCCCCTGTACCCGCGACTATCC | | |
LA1 | CCGCAACCTGGTGCCCATGG | advanced stage gp64 | 139 |
LA2 | CGTTTGGGTTGCGCAGCGGG | | |
LA3 (probe) | TTCTTCTGGGACGCCAACGACATCTACCGCATCTTCGCCG | | |
IE1a | AGATCGCCTGGAGACGCCAT | Early exon 1 | 139 |
IE1b | GGAATCCGCGTTCCAATGCA | | |
IE2a | ATGGAGTCCTCTGCCAAGAG | Early exon 2 | 72 |
IE2b | CCGTGGCACCTTGGAGGAAG | | |
IE3a | GTGACCAAGGCCACGACGTT | Early exon 3 | 167 |
IE3b | TCTGCCAGGACATCTTTCTC | | |
IE4a | ACAGATTAAGGTTCGAGTGC | Early exon 4 | 179 |
IE4b | CAATACACTTCATCTCCTCG | | |
IE4c | TTACCAAGAACTCAGCCTTC | Early exon 4 | 158 |
IE4d | GTGCGTGAGCACCTTGTCTC | | |
IE4e | TATACCCAGACGGAAGAGAAATTCA | Early exon 4 | 426 |
IE4f | ATAAGCCATAATCTCATCAGGGGAG | | |
Pp1a | TAGCGCGCATACATCCCGAGTACAT | Pp71 gene | 316 |
Pp1b | ATGACGTTGCTCCGTGGAAAGAGACC | Pp71 | |
3. Nested PCR amplification: ① The reaction mixture composition is the same as (2), except the primers are IE2a and IE4b (covering the entire exon 3, totaling 721b), each at 100 pmol. Perform amplification at 94°C for 60s, 52°C for 150s, and 72°C for 480s, for 20 cycles. ② Take 2μl of the above amplification product, add 100 pmol each of primers IE3a and IE3b, and supplement with other reagents. Then, perform amplification at 94°C for 60s, 52°C for 150s, and 72°C for 180s, for 20 cycles.
(5) Product identification
The analysis of amplification products can be performed using solid-phase (filter membrane) hybridization and liquid-phase hybridization assays.
1. Solid-phase hybridization:
① The pre-hybridization solution is 3×SSPE, 5×Denhardt, 0.5% SDS, and 25% formamide. The filter membrane containing the amplification product is pre-hybridized at 42°C for 30–60 min. ② Add the labeled probe (10 cpm/μg, 2 ng/ml) and hybridize for 30–60 min. ③ Wash the filter membrane three times at room temperature with 0.2×SSPE and 0.1% SDS, 5 min each time; wash once at 60°C for 10 min; then wash once more at room temperature for 5 min. ④ Autoradiography.
2. Liquid-phase hybridization and electrophoresis analysis:
① Take 1/10 volume of the amplification product and mix with 0.5–1.0 pmol of the end-labeled probe. ② Add a solution with a final concentration of 150 mmol/L NaCl, 10 mmol/L sodium phosphate, and 1 mmol/L EDTA, bringing the total volume to 20μl. ③ Heat at 95°C for 10 min, then at 56°C for 60 min. ④ Centrifuge for 10 s, add sample buffer, and perform electrophoresis in an 8% polyacrylamide gel. ⑤ After electrophoresis, stain with ethidium bromide, then expose to X-ray film for autoradiography.
The HCMV DNA molecule is very large, and its base sequence has not yet been fully elucidated. Although its DNA exhibits restriction endonuclease fragment length polymorphism, the discreteness of its genome remains unclear. PCR amplification using a single primer pair can identify 90% of HCMV wild-type isolates, while employing two or more primer pairs targeting different HCMV sequences can detect nearly all viral strains. Therefore, depending on the situation, PCR testing can be performed using two or more primer pairs located in different regions of the genome.
During the preparation of HCMV template DNA, small DNA fragments are sometimes generated, which can lead to a certain level of background products during PCR reactions, thereby affecting result interpretation. In such cases, nested PCR can be employed. The basic principle involves a two-step amplification of the target DNA: first, a pair of primers is used to amplify a long DNA fragment containing the target DNA; then, a small amount of the amplified product is taken and subjected to a second amplification using primers specific only to the target DNA. By controlling the number of amplification cycles in each step, false positives caused by small DNA fragments can be prevented. This method also helps avoid false-negative results.
PCR detection of HCMV specimens exhibits high sensitivity. From the supernatant of HCMV-infected tissue cultures, DNA molecules equivalent to dozens of viruses or 1–5 PFU viral DNA sequences can be detected. Southern blot hybridization analysis of the amplified products using non-radioactive oligonucleotide probes can detect HCMV DNA sequences at a level of 1 pg in urine specimens. With this system, as few as one viral genome among 4×10^4 cells can be detected, representing a 2×10^3-fold improvement over dot blot hybridization.
PCR technology holds clinical value for detecting HCMV infections because viral DNA in bodily fluids appears earlier than clinical symptoms or serological evidence of infection, serving as an early indicator of HCMV infection. Since HCMV can be transmitted through intraplacental infection, birth canal infection, and other routes—and infected newborns have a high mortality rate—early diagnosis via PCR and timely treatment measures are crucial for eugenics and prenatal care. This method can also determine whether donors in organ or tissue transplants are associated with HCMV and many severe conditions. Furthermore, PCR detection offers stable indicators and enables semi-quantitative analysis, making it a valuable tool for evaluating the efficacy of various antiviral drugs.
bubble_chart Treatment Measures
The treatment of cytomegalovirus infection can involve various antiviral agents such as GCV, anti-cytomegalovirus immunoglobulin preparations, interferon, and transfer factors. However, these drugs do not address the root cause, and the virus often rebounds latently after discontinuation of the medication. Given that this virus may serve as one of the disease causes of Acquired Immune Deficiency Syndrome, scholars worldwide are dedicated to research aimed at controlling its infection. Recently, American researchers have developed two live vaccines, which have shown promising results in initial trials. One is derived from the AD169 strain, and the other is made from the TOWn strain. After parenteral administration, both have demonstrated significant efficacy against cytomegalovirus, with elevated CMV antibody levels leading to enhanced immune function.





