| disease | Anomalous Origin of the Coronary Artery |
Coronary artery anomalous origin commonly originates from the pulmonary artery and the main coronary artery. Origins from the common carotid artery and the innominate artery are extremely rare and are often associated with severe cardiac malformations. Brooks first reported two cases in 1885. The most common anomalous origin from the pulmonary artery is the left coronary artery. Additionally, there are rare cases of the right coronary artery originating from the pulmonary artery, both coronary arteries originating from the pulmonary artery, the left circumflex coronary artery originating from the pulmonary artery, and accessory or conus coronary arteries originating from the pulmonary artery. Cases where both coronary arteries originate from the pulmonary artery result in death within days after birth due to severe myocardial ischemia and hypoxia, making clinical diagnosis extremely rare. Anomalous origin of the left coronary artery from the pulmonary artery occurs when the left coronary artery arises from the pulmonary artery, but its branch distribution and course are normal. The incidence is only 1 in 300,000 live births, accounting for 0.26% of congenital heart diseases. Bland, White, and Garland first described the clinical manifestations of this condition in 1933.
bubble_chart Pathological Changes
Coronary stirred pulse ectopically originating from the pulmonary stirred pulse (Figure 5) is the most common.
Figure 5 Coronary stirred pulse ectopically originating from the pulmonary stirred pulse
1. Left coronary stirred pulse originating from the pulmonary stirred pulse; 2. Left coronary stirred pulse originating from the pulmonary stirred pulse; 3. Left and right coronary stirred pulse originating from the pulmonary stirred pulse; 4. Accessory coronary stirred pulse originating from the pulmonary stirred pulse; 5. Circumflex coronary stirred pulse originating from the pulmonary stirred pulse.
Pathophysiology: The left coronary artery originates ectopically from the pulmonary artery. The pathophysiological effects depend on the pressure difference between the systemic and pulmonary circulations, as well as the extent and abundance of collateral circulation between the left and right coronary arteries. During the fetal and neonatal periods, the pressures and oxygen content in the left and right ventricles are equal, and the resistance of the pulmonary circulation is similar to that of the systemic circulation. Therefore, the left coronary artery originating from the pulmonary artery can receive perfusion pressure and oxygen supply equivalent to that from the aorta, posing no impact on fetal growth and development. Within 7–10 days after birth, pulmonary vascular resistance decreases, and pulmonary artery pressure normalizes. Concurrently, the oxygen saturation of pulmonary artery blood drops to around 70%, and the hematocrit decreases from 70–80% before birth to approximately 40% by 3 months postpartum, accompanied by a reduction in hemoglobin levels. As a result, the ectopically originating left coronary artery not only experiences a drop in perfusion pressure to 2.7–6.7 kPa (20–50 mmHg) but also a significant reduction in the oxygen content of the perfused blood, leading to myocardial hypoxia in the left coronary artery's perfusion zone. In cases of left ventricular hypertrophy, the subendocardial myocardium undergoes widespread fibrosis due to ischemia and hypoxia. The survival of the infant after birth depends on the development of collateral circulation between the left and right coronary arteries. If collateral circulation is well-developed, the infant may survive; if insufficient, myocardial infarction and death will inevitably occur. If collateral circulation is excessively abundant, blood flow from the right coronary artery (originating from the aorta) may enter the left coronary artery and pulmonary artery via collateral channels, creating a left-to-right shunt. This leads to congestive heart failure and coronary steal syndrome. Approximately 80–90% of patients die from congestive heart failure or myocardial infarction within the first year of life. Only a minority of patients, where collateral circulation between the left and right coronary arteries is robust and most of the myocardium—including the diaphragmatic surface of the left ventricle, the majority of the interventricular septum, and the lateral wall of the left ventricle—receives blood supply from the right coronary artery, may survive into adulthood.
bubble_chart Clinical ManifestationsWithin the first month after birth, sick infants may show no abnormal symptoms. However, between 2 to 3 months after birth, they may begin to exhibit myocardial ischemia and hypoxia. Symptoms such as shortness of breath triggered by feeding or crying, dysphoria, restlessness, pale or cyanotic lips, great dripping sweating, lack of strength, increased heart rate, cough, and wheezing may occur due to heart colicky pain and heart failure. In rare cases with very rich collateral circulation in the left and right coronary stirred pulse, symptoms of heart colicky pain and chronic congestive heart failure may be delayed until around 20 years of age. These cases often present with continuous murmurs in the precordial area, and mitral valve insufficiency is also more severe.
Physical examination: Poor growth and development, thin and small stature, failure to gain weight, increased respiratory rate, enlarged cardiac dullness, accelerated heart rate, hepatomegaly, jugular vein distension, pulmonary rales, and other signs of heart failure. A systolic murmur caused by mitral valve insufficiency can be heard in the apical region. In cases with rich collateral circulation in the coronary stirred pulse, a soft continuous murmur may be heard in the precordial area.
bubble_chart Auxiliary Examination
Chest X-ray: Shows significant enlargement of the cardiac shadow, fullness and bulging of the left heart border, a rounded and blunt cardiac apex protruding outward and downward to the left axillary region, and overlapping the spine posteriorly. The lung fields exhibit vascular congestion, but with weaker pulsations.
Electrocardiogram (ECG): Often shows signs of anterolateral myocardial infarction, with Qr waves and T-wave inversion in leads L1 and AVL. Deep Q waves are present in V5
Right heart catheterization: In cases with abundant collateral circulation of the coronary {|###|} artery, blood from the right coronary {|###|} artery flows through collateral circulation into the left coronary {|###|} artery and then into the pulmonary {|###|} artery, resulting in increased oxygen content in the pulmonary {|###|} artery blood. A left-to-right shunt can be detected at the pulmonary {|###|} artery level, and pulmonary {|###|} artery pressure may also be elevated.
Echocardiography: Reveals left ventricular dilation and significantly weakened myocardial contractility. Cross-sectional echocardiography and pulsed Doppler ultrasound can demonstrate the anomalous origin of the left coronary {|###|} artery from the pulmonary {|###|} artery.
Serum enzyme assays may show elevated levels of creatine phosphokinase, lactate dehydrogenase, and aspartate aminotransferase.
Radionuclide myocardial imaging: Myocardial imaging with 201TI can reveal non-visualization of the myocardium in the anterolateral damaged area of the heart.
Selective cardiac angiography: Cardiac angiography is a reliable method for confirming the anomalous origin of the coronary {|###|} artery. Aortic angiography and selective right coronary {|###|} angiography show only the right coronary {|###|} artery originating from the aorta, with significant dilation of the right coronary {|###|} artery. Contrast medium fills the left coronary {|###|} artery retrogradely and then refluxes into the pulmonary {|###|} artery. Selective left ventricular angiography often reveals left ventricular cavity dilation, significantly weakened left ventricular contractility, and impaired motion of the left ventricular anterior wall. Selective left ventricular angiography also aids in diagnosing mitral regurgitation. In some cases, the left coronary {|###|} artery may become visible when contrast medium is injected into the pulmonary {|###|} artery.
bubble_chart Treatment Measures
The anomalous origin of the left coronary artery from the pulmonary artery has a very poor natural prognosis, with approximately 65% of patients dying from left heart failure within the first year of life, most within the first two months. Wesselhoeft reported that 80% of 60 infant cases died within the first year. Patients with well-developed collateral circulation may survive into adulthood, but often succumb to chronic congestive heart failure or sudden death due to progressive left ventricular ischemic changes. Therefore, surgical treatment should be pursued once the diagnosis is confirmed.
**History of Surgical Treatment**: In 1955, Potts, Kittle, Paul, and Robbins performed procedures such as aortopulmonary fistula creation, pulmonary artery ligation, and intrapericardial talc poudrage, but the outcomes were unsatisfactory. In 1959, Sabiston successfully performed ligation of the left coronary artery. In 1966, Cooley used the great saphenous vein for aortocoronary bypass grafting. In 1968, Meyer performed a left subclavian artery-left coronary artery anastomosis. In 1974, Neches successfully transplanted the left coronary artery directly into the ascending aorta or connected it using a segment of the free left subclavian artery. Also in 1974, Hamilton and Takeuchi separately used pericardial membrane and pulmonary artery wall to construct conduits within the pulmonary artery, anastomosing them to the ascending aorta and the left coronary artery orifice.
**Surgical Procedures**:
1. **Ligation of the Left Coronary Artery**: A left anterolateral thoracotomy through the fourth intercostal space is performed. The pericardium is incised anterior to the phrenic nerve to expose the dilated left coronary artery. The artery is dissected free near the pulmonary artery, and a non-traumatic vascular clamp is applied for several minutes to observe ECG changes and whether the coronary artery pressure remains high. If no ECG changes occur and the coronary pressure does not drop, the artery is doubly ligated with silk sutures. This procedure is simple but only suitable for cases with abundant collateral circulation and significant left-to-right shunting. Post-ligation, the left-to-right shunt and coronary steal phenomenon disappear, myocardial blood supply improves, heart failure resolves, cardiac size decreases, and normal growth and weight gain are observed. However, the mortality rate for infant cases can be as high as 50%, while it significantly decreases for patients over two years old.
2. **Left Subclavian Artery-Left Coronary Artery Anastomosis**: This procedure can be performed with or without cardiopulmonary bypass. Without bypass, a left anterolateral thoracotomy through the fourth intercostal space is used to dissect the left coronary artery root and left subclavian artery. The left subclavian artery and its vertebral branches are ligated and divided at the thoracic apex, and the proximal segment is flipped downward. After ligating the left coronary artery root, the proximal subclavian artery is anastomosed end-to-side to the left coronary artery.
With cardiopulmonary bypass, a median sternotomy is performed. The pericardium is opened, and a single venous cannula is placed in the right atrium via the right atrial appendage, while an arterial cannula is inserted into the ascending aorta. After establishing bypass, the left coronary artery is dissected free, and its orifice, along with the adjacent pulmonary artery wall, is excised from the pulmonary artery, which is then sutured or patched. The left subclavian artery is ligated and divided, and its proximal segment is flipped downward for end-to-end anastomosis with the left coronary artery. Postoperative outcomes are favorable, with some cases showing long-term patency on angiography. However, if the subclavian artery is too short, anastomosis tension or twisting may lead to poor patency, limiting its clinical application.
3. Ascending aorta stirred pulse-left coronary stirred pulse anastomosis A surgical procedure that connects the ectopically originated left coronary stirred pulse to the ascending aorta stirred pulse, establishing a normal coronary stirred pulse blood supply and restoring the physiological state of coronary stirred pulse perfusion. In recent years, with the advancement of extracorporeal circulation technology, this procedure has been increasingly promoted and applied. There are several methods for this type of surgery, most of which require the use of extracorporeal circulation combined with moderate or deep hypothermia and myocardial protection measures such as cold cardioplegia.
(1) Ectopic origin of the left coronary artery transplanted into the ascending aorta: A median sternotomy is performed, and after establishing extracorporeal circulation, the pulmonary artery is dissected. The anterior wall of the pulmonary artery is transversely incised near the pulmonary valve to expose the orifice of the left coronary artery. The orifice of the left coronary artery, along with a portion of the surrounding pulmonary artery wall, is excised. The pulmonary artery is then transected transversely, and the proximal segment of the left coronary artery is dissected. A small window is made at the root of the ascending aorta, and the excised left coronary artery orifice with its surrounding pulmonary artery wall is anastomosed end-to-side to the small incision at the root of the ascending aorta. The cut end of the pulmonary artery is then directly sutured (Figure 1). This surgical design is reasonable, meets normal physiological requirements, and yields good therapeutic outcomes. However, if the length of the left coronary artery is insufficient, causing excessive tension after transplantation into the ascending aorta, alternative surgical methods must be considered.
(1) Incision of the pulmonary artery
(2) Excision of the left coronary artery
(3) Transplantation of the left coronary artery into the ascending aorta
(4) Completion of anastomosis
(5) Suturing of the pulmonary artery
Figure 1: Ectopic transplantation of the left coronary artery into the ascending aorta
(2) Aortocoronary bypass grafting using the great saphenous vein: In 1966, Cooley; in 1973, El Said; in 1974, Neches; and in 1980, Arciniegas, among others, successively used segments of reversed autologous or homologous great saphenous veins, subclavian arteries, or Dacron artificial blood vessels to connect the ascending aorta to the cut end of the left coronary artery. For the graft, one end is anastomosed end-to-side to the ascending aorta, and the other end is anastomosed end-to-side or end-to-end to the left coronary artery. For end-to-side anastomosis, the origin of the left coronary artery is dissected near the pulmonary artery wall, doubly ligated with sutures, and the left coronary artery trunk is longitudinally incised. The great saphenous vein is then passed anterior to the pulmonary artery trunk and anastomosed end-to-side to the left coronary artery (Figure 2). For end-to-end anastomosis, the orifice of the left coronary artery, along with its surrounding pulmonary artery wall, is excised from the pulmonary artery, and the pulmonary artery wall incision is sutured. The proximal segment of the left coronary artery is then dissected and anastomosed end-to-end to the great saphenous vein. End-to-end anastomosis is technically less challenging than end-to-side anastomosis. End-to-side anastomosis can be performed without extracorporeal circulation. Due to the small size and thin walls of the coronary arteries and great saphenous veins in infants and young children, complications such as obstructive lesions or vascular dilation are more likely postoperatively. The long-term patency rate and therapeutic outcomes require further follow-up observation.
Figure 2 Main artery-left coronary artery bypass grafting with great saphenous vein
(1) The left coronary artery originates from the pulmonary artery, with coronary circulation flowing retrograde into the pulmonary artery; (2) Ligate the origin of the left coronary artery; (3) Perform a main artery-left coronary artery bypass grafting using the great saphenous vein; (4) Complete the grafting
(3) Intrapulmonary tunnel procedure: This surgical technique has only been in clinical use for slightly over a decade. Its advantage lies in eliminating the need to dissect and mobilize the left coronary artery or perform the technically challenging incision and suturing on small coronary arteries. It is particularly suitable for cases where the left coronary artery ostium is located on the left lateral wall of the pulmonary artery, resulting in a shorter length.
A median sternotomy is performed, the pericardium is opened, and extracorporeal circulation is established with moderate or deep hypothermia. The left lateral wall of the pulmonary artery is incised near the pulmonary valve to expose the left coronary artery ostium. Then, adjacent sections of the ascending aorta and pulmonary artery walls are each excised to create 5-6mm diameter circular openings. These openings are directly sutured together to form an artificial aortopulmonary fistula. Above this fistula, a transverse incision is made in the pulmonary artery, extending to the left wall where it connects with a lower transverse incision, creating a parallel rectangular flap from the anterior pulmonary artery wall. This flap is then sutured to the posterior pulmonary artery wall, with its right and left ends attached around the aortopulmonary fistula and left coronary ostium respectively. This allows blood to flow from the ascending aorta through the aortopulmonary fistula into the pulmonary artery lumen, then via the anterior wall tunnel into the left coronary artery. The defect in the anterior pulmonary artery wall is covered with a pericardial or Dacron patch. Alternatively, the intrapulmonary tunnel can be constructed using a pericardial patch (Hamilton, 1979) or a free segment of autologous subclavian artery (Arciniegas, 1980) instead of the anterior pulmonary artery wall (Figure 3). The main factors affecting surgical mortality are disease severity and patient age. For patients with preoperative cardiac function below class III, mortality is under 20%, while those requiring emergency surgery with class IV function face up to 70% mortality. Arciniegas reported 19 cases: among 12 infants under 1 year old, 2 died, while all 7 patients aged 1-2 years survived. Due to the rarity of anomalous left coronary artery origin from pulmonary artery and relatively recent development of surgical treatments, long-term follow-up data remains limited. Kirklin and Barratt-Boyes followed 19 survivors for up to 19 years, with only 1 death - 17 patients achieved class I cardiac function postoperatively, and 1 reached class II. Significant left ventricular size reduction and marked improvement in myocardial ischemia symptoms were observed. Most cases with moderate mitral regurgitation due to ischemia retained apical systolic murmurs postoperatively.
Figure 3 Pulmonary stirred pulse internal channel procedure
(1) Pulmonary stirred pulse incision; (2) Main stirred pulse-pulmonary stirred pulse anastomosis; (3) The anterior wall flap of the pulmonary stirred pulse is sutured with the posterior wall of the pulmonary stirred pulse to form a channel; (4) The defect in the anterior wall of the pulmonary stirred pulse is repaired with a pericardial patch
Right coronary stirred pulse ectopically originating from the pulmonary stirred pulse Right coronary stirred pulse ectopically originating from the pulmonary stirred pulse is far less common than the left coronary stirred pulse. By 1979, only 17 cases had been reported (LeBerg et al.). Most cases do not present clinical symptoms and are diagnosed only during autopsy. Due to the thin wall and low tension of the right ventricle, collateral circulation can form between the two coronary stirred pulses, maintaining myocardial oxygen supply in the area supplied by the right coronary stirred pulse. Infants do not present clinical symptoms, and growth and development are normal. In adulthood, a few patients may develop heart failure or sudden death. Ascending main stirred pulse and pulmonary stirred pulse angiography can reveal the ectopically originating right coronary stirred pulse, thus confirming the diagnosis.
The treatment involves performing surgery under cardiopulmonary bypass, where the opening of the right coronary stirred pulse along with a portion of the surrounding pulmonary stirred pulse wall is excised from the pulmonary stirred pulse and transplanted into the anterior wall of the root of the ascending main stirred pulse. Because the right coronary stirred pulse is relatively long and originates from the anterior wall of the pulmonary stirred pulse near the ascending main stirred pulse, the transplantation procedure is relatively straightforward (Figure 4).
(1) The right coronary stirred pulse is excised from the pulmonary stirred pulse wall
(2) Implanted into the ascending main stirred pulse
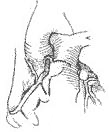
(3) Suturing the pulmonary stirred pulse incision
Figure 4 Right coronary stirred pulse implantation into the main stirred pulse






