| disease | Hypertrophic Obstructive Cardiomyopathy |
Hypertrophic obstructive cardiomyopathy was once referred to as subaortic muscular obstruction. In 1952, Davies reported that five out of nine siblings in one family had this disease, with three cases resulting in sudden death. In 1958, Teare described severe hypertrophy of the ventricular septum, where its thickness far exceeded that of the left ventricular free wall. The myocardial cells were thick and short, arranged in a disordered manner, with abundant lateral connections between cells, termed asymmetric ventricular septal hypertrophy. After 1960, it was recognized as a type of primary cardiomyopathy, accounting for about 20% of all cardiomyopathies, and was thus called idiopathic obstructive cardiomyopathy, idiopathic hypertrophic subaortic stenosis, or hypertrophic obstructive cardiomyopathy. Approximately 30% of cases have a family history, suggesting possible genetic factors. The onset can occur from infancy to over 60 years of age, but it is most commonly seen between the ages of 10 and 30, indicating it may be a congenital malformation or acquired later in life. Starting in 1960, Goodwin, Kelly, Morrow, Brockenbrough, Braunwald, Wigle, and others successively developed surgical treatments for this condition.
bubble_chart Pathological Changes
The severity of left ventricular obstruction in hypertrophic obstructive cardiomyopathy varies. The most typical lesion is marked hypertrophy in the upper part of the ventricular septum. When the septum is longitudinally incised, the hypertrophic myocardium bulges into the left and right ventricular cavities. The thickest part of the ventricular septum lies below the free edge of the anterior mitral valve leaflet, where localized fibrosis and thickening of the endocardium occur due to repeated collision with the leaflet (Figure 1). The thickness of the hypertrophic ventricular septum gradually decreases upward (toward the aortic valve annulus) and downward (toward the apex). The lower segment of the left ventricular outflow tract obstruction is located between the hypertrophic ventricular septum and the free edge of the anterior mitral valve leaflet. During systole, the hypertrophic septum protrudes into the ventricular cavity, approaching the anteriorly displaced mitral valve leaflet, causing narrowing of the left ventricular outflow tract, sometimes accompanied by insufficiency. In early systole, the degree of outflow tract obstruction is milder, allowing more ventricular output. The anterolateral free wall and apical regions of the left ventricle show uniform hypertrophy, while the posterior wall is less thickened. The ratio of ventricular septum thickness to posterior wall thickness can reach 3:1, and the left ventricular cavity is often small. If the mid-segment of the ventricular septum is thickened, the ventricular cavity may appear hourglass-shaped. In advanced stages, due to myocardial infarction or prolonged grade III heart failure, the left ventricle may dilate. The left atrial cavity is often enlarged, with thickened atrial walls and anterior mitral valve leaflets, which may be accompanied by chordae tendineae rupture or congenital anomalies. The right ventricle may develop outflow tract obstruction due to protrusion of the hypertrophic septum. In long-standing cases, the free wall of the right ventricle may thicken due to obstructive lesions or elevated pulmonary circulation pressure. The coronary artery branches supplying the ventricular septum and ventricular walls often exhibit thickened walls and narrowed lumens, potentially leading to transmural myocardial obstruction.
bubble_chart Clinical ManifestationsThe clinical symptoms include dyspnea after exertion, syncope or dizziness, and colicky pain after activity, similar to those of {|###|}stirred pulse valve stenosis. Approximately 10% of cases experience palpitations or systemic embolism due to paroxysmal or persistent atrial fibrillation. In advanced stage cases, congestive heart failure, orthopnea, and pulmonary edema occur.
Common signs include enhanced apical impulse, displacement to the left and downward, often with a lifting impulse or double impulse. A systolic intermediate stage [second stage] ejection murmur can be heard at the lower left sternal border or apex, radiating to the base of the heart, often accompanied by a tremor. In cases with mitral regurgitation, a holosystolic murmur is present at the apex, along with splitting of the second heart sound, and the third or fourth heart sound may also be audible. However, systolic ejection clicks are not heard. The peripheral {|###|}stirred pulse has a stronger percussion wave and a smaller dicrotic wave, resembling a water-hammer pulse.
bubble_chart Auxiliary Examination
Chest X-ray: Enlargement of the cardiac shadow, with left ventricular enlargement, but no signs of ascending aortic pulse dilation or valvular calcification. In advanced cases, the left atrium and right ventricle may also enlarge, with pulmonary vascular congestion.
Electrocardiogram: Shows left ventricular hypertrophy and strain, sometimes with abnormal Q waves in leads aVL and I. Some cases may present with complete right bundle branch block, left bundle branch block, or left anterior hemiblock, as well as left atrial hypertrophy.
Cardiac catheterization: Right heart catheterization may reveal elevated pulmonary artery pressure or signs of right ventricular outflow tract obstruction. Left heart catheterization shows a significant increase in left ventricular end-diastolic pressure and a systolic pressure gradient between the left ventricular cavity and the outflow tract. The aortic or peripheral arterial pressure waveform exhibits a rapid upstroke with a double peak, followed by a slow descent. Post-extrasystolic aortic pulse pressure decreases. Administration of nitroglycerin, amyl nitrite, isoproterenol, digitalis, as well as physical exertion and the Valsalva maneuver, can enhance myocardial contractility, worsen left ventricular outflow tract obstruction, and lead to increased murmur intensity and a higher systolic pressure gradient.
Left ventriculography can also determine the presence of mitral regurgitation. Adult patients should undergo coronary angiography to assess for coronary artery disease.
Echocardiography: Demonstrates significant thickening of the left ventricular wall, with the ventricular septum more hypertrophied than the posterior ventricular wall. The left ventricular cavity is small, with outflow tract narrowing and anterior displacement of the anterior mitral leaflet during systole.
bubble_chart Treatment Measures
Hypertrophic obstructive cardiomyopathy can present symptoms at any age, with the most common onset occurring around 20 years old. Among cases diagnosed via cardiac catheterization, only 10% of those under 10 years old exhibit severe symptoms, while this proportion increases to 70% in those over 50. Some cases may remain stable for years or progressively worsen over time. The onset of atrial fibrillation often leads to congestive heart failure or systemic embolism. Among untreated cases with clinical symptoms and arrhythmias, approximately 15% die within 5 years, and 25% die within 10 years. Most patients experience sudden death, with only a minority succumbing to heart failure or infective endocarditis. Surgical treatment is indicated for patients with significant clinical symptoms unresponsive to medical therapy and a resting systolic pressure gradient between the left ventricular cavity and outflow tract exceeding 6.6 kPa (50 mmHg). The procedure involves resection of the hypertrophied ventricular septum to relieve obstruction.
(1) Combined transaortic and left ventricular approach for myocardial resection: A median sternotomy is performed, and cardiopulmonary bypass with hypothermia is established. A decompression drain is placed in the left atrium. The ascending aorta is clamped, and cold cardioplegic solution is infused at the root with local myocardial cooling. The ascending aorta is transversely incised at its root, and the right coronary cusp is retracted forward with a hook. A rounded scalpel is used to resect a U-shaped portion of the hypertrophied myocardium from the anterior ventricular septum, starting below the right coronary cusp and extending leftward to below the junction of the right and left coronary cusps. Care is taken to avoid extending the incision rightward to prevent injury to the left bundle branch and complete heart block. The rectangular myocardial segment is elongated downward under direct vision, but the incision should not be too deep. A separate oblique incision, approximately 4 cm long and parallel to the lowest diagonal branch, is made in the lower anterior left ventricular wall to access the left ventricular cavity below the anterior papillary muscle. Through this incision, the anterior leaflet is retracted toward the left side of the ventricular septum, and hypertrophied myocardium is resected from below upward with a scalpel, connecting with the myocardial segment removed via the aortic approach. The entire hypertrophied myocardial segment is then excised, with an incision depth of about 15–20 mm. Myocardial fragments are trimmed to prevent embolism. The myocardial incision is closed with full-thickness interrupted sutures, and the aortic incision is sutured (Figure 1). Residual air is evacuated from the left ventricular cavity and aorta, the aortic clamp is removed, and rewarming is initiated. Cardiopulmonary bypass is discontinued once cardiac contractions are strong.
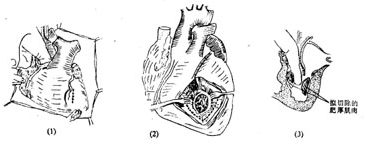
Figure 1: Resection of hypertrophied myocardium via combined transaortic and left ventricular approach
(1) The surgeon’s left index finger is inserted through the aorta into the left ventricular cavity to guide the left ventricular incision; (2) The hypertrophied myocardium to be resected; (3) The extent of resection for the hypertrophied myocardium in the left ventricular wall and ventricular septum.
(2) Transaortic ventricular septal myectomy and myotomy: Cardiopulmonary bypass is established, and myocardial protection measures are implemented. The aorta is clamped, and a transverse incision is made at the root of the ascending aorta. The right coronary cusp is retracted to expose the ventricular septum. Two parallel incisions are made in the upper ventricular septum below the right coronary cusp using a small rounded scalpel. To improve exposure during the lower ventricular septal incision, the right ventricular free wall can be pressed to shift the septum toward the left ventricular cavity. The rectangular hypertrophied myocardial tissue between the two parallel incisions is then excised. The ventricular septal incision is manually pressed to deepen and widen the groove. Myocardial fragments are removed, and the aortic incision is sutured. Air is evacuated from the left ventricular cavity and aorta, and the aortic clamp is removed. Rewarming is continued until the body temperature reaches above 35°C, and cardiopulmonary bypass is discontinued once cardiac contractions are strong. If the resection of the hypertrophied ventricular septum is deemed insufficient, a left ventricular approach can be added for complete resection (Figure 2).
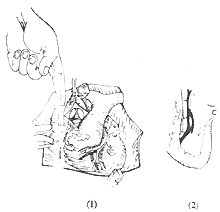
Figure 2 Ventricular Septal Myectomy
(1) After establishing extracorporeal circulation, insert the surgeon's finger and a small knife through the aortic pulse incision; (2) Make a longitudinal incision into the hypertrophic myocardium of the ventricular septum.
Therapeutic effect: The surgical mortality rate is approximately 10%. Common causes of death include low cardiac output and bleeding from the left ventricular incision. Postoperatively, about 5% of cases develop complete heart block, with a higher incidence of left or right bundle branch block. Additionally, a small number of patients experience perioperative myocardial infarction, ventricular septal rupture, left ventricular aneurysm, or iatrogenic aortic or mitral valve insufficiency.
In cases where hypertrophic myocardium of the ventricular septum is thoroughly resected, postoperative symptoms disappear or significantly improve, the systolic pressure gradient is eliminated, and aortic pressure waveforms return to normal. Follow-up echocardiography and selective left ventriculography show an enlarged left ventricular cavity and the disappearance of systolic anterior motion of the mitral valve, though atrial fibrillation persists. Approximately 90% of patients achieve postoperative cardiac function improvement to NYHA class 1-2.
Long-term follow-up shows that 70% of patients survive for more than 10 years postoperatively, and 50% survive for over 15 years. The main causes of death are congestive heart failure or severe arrhythmias, as well as cerebral embolism or myocardial infarction due to atrial fibrillation. The incidence of sudden death is about 25%, significantly lower than in untreated patients.





