| disease | Neonatal Respiratory Distress Syndrome |
| alias | Hyaline Membrane Disease, Hyaline Miembrane Disease, Neonatal Respiratory Distress Syndrome,NRDS |
Neonatal respiratory distress syndrome (NRDS) can be broadly and narrowly defined. The former refers to any respiratory distress symptoms, regardless of the disease cause, that can be named as such, while the latter refers to respiratory distress syndrome (RDS) caused by a lack of pulmonary surfactant. This article mainly discusses the latter type of NRDS. It primarily occurs in premature infants, with progressive dyspnea as the main clinical manifestation, and is pathologically characterized by the presence of eosinophilic hyaline membranes and atelectasis, hence it is also known as hyaline membrane disease.
bubble_chart Pathogenesis
Neonatal respiratory distress syndrome (NRDS) is caused by a deficiency of pulmonary surfactant (PS). Due to the surface tension at the interface between alveoli and air, the absence of surfactant leads to the compression of alveoli, gradually forming atelectasis, which progressively enlarges. Blood flowing through the atelectatic region returns to the heart without gas exchange, creating an intrapulmonary shunt. Consequently, the blood PaO2 decreases, oxygenation is reduced, and the body's metabolism can only proceed under hypoxic conditions, leading to acidosis. During acidosis, pulmonary vasospasm increases pulmonary vascular resistance, raising right heart pressure, and sometimes even reopening the stirred pulse catheter, forming a right-to-left shunt. In severe cases, 80% of the cardiac output becomes shunt volume, leading to significant cyanosis in the infant. As pulmonary blood flow decreases, pulmonary perfusion becomes insufficient, and hypoxia increases vascular wall permeability, causing plasma contents, including proteins, to extravasate. The deposition of fibrin forms a hyaline membrane in the lungs.
【Predisposing Factors of NRDS】1. Premature infants
Fetal type II alveolar cells can produce PS by 22-24 weeks of gestation, but the amount is small and rarely transferred to the alveolar surface. As gestational age increases, PS synthesis gradually increases. Therefore, the more premature the infant, the less PS in the lungs, and the higher the incidence of RDS. Between 24-30 weeks of gestation, various hormones have the greatest effect on promoting lung maturation, making this the optimal stage for prenatal prevention. After 32-34 weeks, the influence of hormones on lung maturation is less significant, and after 35 weeks, PS rapidly enters the alveolar surface. Premature infants continue lung development after birth, and the PS produced within 72-96 hours after birth is generally sufficient to maintain normal respiration. Therefore, supplementing PS during the deficiency phase can help premature infants overcome the critical period and improve survival rates.
2. Infants of diabetic mothers
High blood sugar in diabetic mothers leads to elevated fetal blood sugar, necessitating increased insulin secretion to meet glucose metabolism needs and convert glucose into glycogen. This results in fetal macrosomia, but the lungs may not mature adequately. Additionally, insulin antagonizes adrenal corticosteroids, affecting lung development.
3. Intrauterine distress and birth asphyxia
Intrauterine distress often occurs in fetuses with placental insufficiency, where chronic hypoxia affects lung development and reduces PS secretion. Birth asphyxia, often caused by difficult delivery, is one of the reasons for RDS in newborns.
bubble_chart Pathological Changes
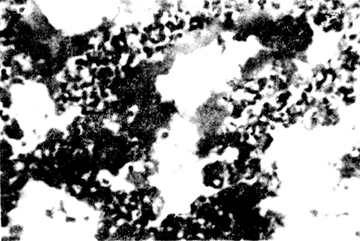
Figure 1 Hyaline membrane disease of the lung
bubble_chart Clinical Manifestations
1. Clinical Symptoms
Most affected infants are premature babies. At birth, their crying may be normal, but they develop respiratory difficulties within 6 to 12 hours, which gradually worsen, accompanied by moaning. Breathing becomes irregular, with intermittent apnea. The complexion turns pale or bluish-gray due to hypoxia, and cyanosis becomes evident after right-to-left shunting, not alleviated by oxygen supply. Severe hypoxia leads to decreased muscle tone in the limbs. Signs include nasal flaring, initial chest bulging followed by depression as atelectasis worsens, most noticeable under the armpits. Soft tissue retraction occurs during inspiration, most pronounced below the rib margin and at the lower end of the sternum. Lung sounds are diminished, and fine moist rales can be heard during inspiration. This condition is self-limiting; survival beyond three days increases lung maturity and recovery prospects. However, many infants develop pneumonia, worsening the condition until infection control improves it. Severe cases often result in death within three days, with the highest mortality on the second day after birth.
2. Blood Generation and Transformation Tests
Due to poor ventilation, PaO2 is low, and PaO2 is elevated. Blood pH decreases due to metabolic acidosis. These three tests can be monitored percutaneously, which is convenient but does not represent the actual blood condition, requiring periodic direct testing of stirred pulse blood. In metabolic acidosis, base excess (BE) decreases, and carbon dioxide combining power drops. During the disease, blood often shows low Na+, K+, and high Cl-, necessitating blood electrolyte tests.
3. X-ray Manifestations
Early in hyaline membrane disease, there is a general decrease in translucency in both lung fields, with uniformly distributed fine granular and reticular shadows, representing small atelectatic alveoli and congested small vessels, respectively. Bronchi show air signs but are easily obscured by the heart and thymus shadows, becoming clear in segmental and peripheral bronchi. If atelectasis extends to the entire lung, the lung fields appear ground-glass, making the air-filled bronchi more visible, resembling bare branched trees, with good chest expansion and normal diaphragm position (Figure 3A B)

Figure 2 Hyaline Membrane
(Electron microscopy, showing vacuolar changes in lamellar bodies within type II cells)
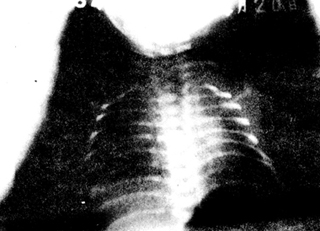
Figure 3A Hyaline Membrane Disease
(Showing granular shadows in lung fields and bronchial air signs)
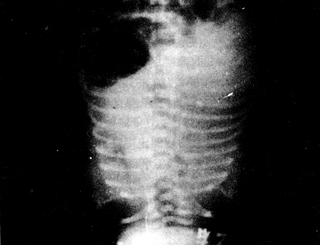
B Hyaline Membrane Disease
(Showing ground-glass shadows in lung fields and bronchial air signs)
bubble_chart Auxiliary Examination
Laboratory tests include the analysis of prenatal intrauterine amniotic fluid and postnatal tracheal aspirates, with the same methods and result interpretation for both. There are multiple testing methods, all of which have relatively high sensitivity and specificity.
I. Generation and Transformation Methods
Thin-layer chromatography (TLC) is generally used. At the beginning of the third trimester, the amounts of PC and S are approximately equal. By 34 weeks of gestation, PC increases rapidly, while S remains relatively stable or slightly decreases, leading to an increase in the L/S ratio. Shortly thereafter (around 35 weeks of gestation), PG begins to appear and rises rapidly once present. Therefore, 34 to 36 weeks of gestation is the optimal period for laboratory testing.
(1) L/S Ratio: An L/S ratio ≥ 2 indicates "lung maturity," 1.5–2 is a transitional or questionable value, and < 1.5 indicates "lung immaturity." If the amniotic fluid is not severely contaminated by meconium or is from vaginal discharge, it has little effect on the test results. The L/S ratio in diabetic pregnant women is often higher, and even if it is > 2, the infant may still develop RDS. Therefore, for diabetic pregnant women, relying solely on one test is insufficient; it should be cross-referenced with other test results (e.g., PG) for greater reliability.
(2) PG: PG can be detected by thin-layer chromatography when it reaches 3% in PS. The presence of PG indicates "lung maturity." It has high sensitivity but lower specificity (approximately 75%).
(3) DPPC Value: A measured value > 500 mg/dl indicates lung maturity. However, about 10% of subjects with DPPC levels of 500–1000 mg/dl still develop NRDS.
II. Foam Test
This is a biophysical measurement method. The principle is that PS promotes the formation and stabilization of foam, while pure alcohol inhibits foam formation. Method: Take 0.5–1.0 ml of amniotic fluid or bronchial secretion, add an equal volume of 95% alcohol, shake vigorously for 15 seconds, and observe the formation of foam around the liquid surface in the test tube after standing for 15 minutes. No foam is (-), ≤ 1/3 of the tube circumference with small foam is (+), > 1/3 to the entire tube circumference with a layer of small foam is (++), and foam in the upper part of the tube is (+++). (-) indicates low PS and can be diagnosed as a deficiency sign, (+) or (++) is questionable, and (+++) indicates high PS. This method is the one-tube foam test. Alternatively, a four-tube foam test can be performed. Refer to Section 3 of Chapter 3 for the physiology and function of amniotic fluid.
bubble_chart Treatment Measures
1. Nursing
Premature infants should receive enhanced nursing care. Place the infant in a temperature-controlled incubator or on a radiant infrared warming bed, and monitor body temperature, respiration, heart rate, transcutaneous TcO2, and TcCO2 using a monitor. Additionally, monitor the mean airway pressure. The environmental temperature should maintain the abdominal skin temperature at 36.5°C or the rectal temperature (core or deep temperature) at 37°C to minimize oxygen consumption. The relative humidity should be around 50%. Regularly clear mucus from the pharynx to keep the airway clear. Pay attention to fluid intake and nutrition; intravenous hyperalimentation can be used until the infant can suck and swallow, then breastfeed.
2. Oxygen Supply and Mechanical Ventilation
To improve hypoxia and reduce anaerobic metabolism, sufficient oxygen must be supplied. Mild cases can use a stuffy nose, mask, or continuous positive airway pressure (CPAP). If FiO2 reaches 0.8 and PaO2 remains below 6.65 kPa (50 mmHg), endotracheal intubation and mechanical ventilation are required. The peak inspiratory pressure should not exceed 2.9 kPa (30 cmH2O), and the mean airway pressure should be <0.98 kPa (<10 cmH2O). The respiratory rate should be 25-30 breaths per minute, with an inspiratory time (I):expiratory time (E) ratio of 1:1 to 2. FiO2 should start high and gradually decrease to 0.4. When weaning off the ventilator, use intermittent mandatory ventilation (IMV) as a transition, with one mandatory breath every 10 breaths. High-frequency ventilation can also be used, with smaller tidal volumes and higher ventilation rates. Due to the short inspiratory time, both peak inspiratory pressure and mean airway pressure are low, and intrathoracic pressure is also low, which is beneficial for venous return. A common method is high-frequency jet ventilation (HFJV), where a nasal tube is inserted 1.5-2 cm into the newborn's nostril, with a driving oxygen pressure (working pressure) of 0.125 kg/cm2, and a jet frequency of 150-300 breaths per minute. Depending on the condition, this can be alternated with conventional stuffy nose oxygen therapy after 1-3 hours, until the blood PaO2 can be maintained above 7.98 kPa (60 mmHg) but not exceeding 11.97-13.3 kPa (90-100 mmHg), then switch to stuffy nose method.
3. Pulmonary Surfactant Replacement Therapy
PS has become a routine treatment for NRDSP. The first dose of natural PS (including porcine and bovine PS) is 120-200 mg/kg, and the second and third doses can be reduced to 100-120 mg/kg, with intervals of about 8-12 hours. Each calculated dose is prepared in 3-5 mg/kg of physiological saline. Temporarily disconnect the endotracheal tube from the ventilator, then directly drip the PS into the lungs through the endotracheal tube. Rotate the infant's position from supine to right lateral and then to left lateral to ensure even distribution of the medication into all lung lobes. If there is a small side channel in the endotracheal tube, the PS can be dripped through this channel to avoid affecting blood oxygen saturation fluctuations. Respiratory distress symptoms can be alleviated within 1-2 hours after administration. If using synthetic Exosurf, the dose is 5 ml/kg, containing DPPC 67 mg/kg, and the effective time appears later, with symptoms improving in about 12-18 hours. Whether natural or synthetic, the earlier PS is used, the better the therapeutic effect. Natural PS does not increase the incidence of allergic diseases later on.
A small number of infants respond poorly to PS treatment for various reasons: ① The lungs of extremely low birth weight infants are not only functionally immature but also structurally immature, accompanied by pulmonary dysplasia. ② Grade III asphyxiated infants respond very poorly. ③ The presence of pulmonary edema (e.g., large left-to-right shunt in PDA) results in a high protein content in the exudate, which antagonizes PS. ④ Accompanying other diseases such as severe pneumonia, thus it is necessary to identify the cause and provide additional treatment.
IV. Stage of Convalescence: Treatment of Patent Ductus Arteriosus (PDA)
Indomethacin can be used, administered in three doses at 12-hour intervals. The initial dose is 0.2 mg/kg, with the second and third doses adjusted based on age: for infants less than 2 days old, each dose is 0.1 mg/kg; for those aged 2 to 7 days, 0.2 mg/kg; and for those older than 8 days, 0.25 mg/kg. The drug can be administered intravenously, and direct infusion into the PDA via a cardiac catheter may enhance efficacy. Oral administration is also possible but less effective. Side effects of indomethacin include reduced renal function, decreased urine output, lowered blood sodium, and elevated blood potassium, which are reversible upon discontinuation. If the medication fails to close the PDA, surgical ligation may be necessary.
V. Antibiotic Therapy
Due to the difficulty in distinguishing hyaline membrane disease from Group B β-hemolytic streptococcal infection, penicillin therapy is often recommended concurrently. The dose is 200,000 to 250,000 units/kg per day, divided into 3-4 doses administered intravenously or intramuscularly.
VI. Fluid Therapy
I. Prenatal Prevention
Refers to the administration of adreno-cortical hormone (ACH) to pregnant women at risk of premature labor during the late stage of pregnancy [third stage] to prevent or alleviate the symptoms of Respiratory Distress Syndrome (RDS) in premature infants after birth. In 1969, Liggins first discovered that intravenous infusion of dexamethasone could promote lung maturation in premature lambs. Similar results were observed in the lungs of other heterologous animals, and this method was gradually applied to pregnant women to promote lung maturation in premature infants. The most commonly used hormones are betamethasone and dexamethasone, as they are more easily transported across the placenta to the fetus compared to other ACHs. The role of ACH is to stimulate the production of phospholipids and small molecular proteins in type II cells of the fetal lung, reduce the permeability of pulmonary capillaries, and decrease pulmonary edema, thereby reducing the incidence of RDS. Even if the disease occurs, the symptoms are milder, and the mortality rate can be reduced. The concentration of oxygen provided during treatment does not need to be too high, which can prevent complications such as bronchopulmonary dysplasia (BPD) and retrolental fibroplasia (ROP). Since hypoxia is alleviated, it should theoretically also reduce the incidence of neonatal necrotizing small intestine colitis and hypoxic-ischemic intracranial hemorrhage.
The preventive dose of ACH for pregnant women; betamethasone or dexamethasone is 24mg each, divided into 2 intramuscular injections, 24 hours apart. The commonly used dose in China is 5-10mg, intramuscular or intravenous, once a day for 3 days. Prevention should be given 7 days to 24 hours before childbirth to ensure the medication has enough time to take effect. ACH prevention does not increase the risk of infection in pregnant women and fetuses, and even if the amniotic membrane ruptures early, it will not further increase the infection rate on the original basis. Intrauterine growth retardation is not a contraindication. The effect of preventing RDS in very low birth weight infants is inconsistent, and it is generally believed that it cannot reduce the incidence of RDS, but the incidence of subependymal germinal matrix hemorrhage in surviving infants may be reduced. The efficacy of ACH is poorer in infants of diabetic pregnant women, children with Rh hemolytic disease, and multiple births.
Although ACH prevention has definite efficacy, 10% of premature infants still develop RDS, so the addition of other hormones is considered to further improve efficacy. Thyroxine has the effect of promoting lung maturation, but due to its difficulty in crossing the placental barrier, it cannot be used clinically. Later, it was found that thyrotropin releasing hormone (TRH) in animal brain tissue has a structure and function similar to thyroxine and can cross the placenta, making it a potential preventive agent. The dose is 0.4mg each time, every 8 hours, for a total of 4 times. Some pregnant women may experience side effects such as nausea, vomiting, and hypertension, and the dose can be reduced to half. With the addition of TRH, the incidence and mortality rate of RDS are further reduced.
II. Postnatal Prevention
It is recommended to administer pulmonary surfactant (PS) to infants within half an hour after birth to prevent or alleviate the symptoms of Respiratory Distress Syndrome (RDS). This is particularly used for infants whose mothers did not receive prenatal prevention. The earlier the prevention, the better the effect. It is best to administer PS through an endotracheal tube before the infant starts breathing or before initiating positive pressure ventilation. This ensures even distribution of PS in the lungs. The preventive effect is manifested in a reduced incidence and mortality rate of RDS, milder symptoms in affected infants, and improved oxygenation. Some infants may not require mechanical ventilation, and the required oxygen concentration and mean airway pressure can be lower. Consequently, the incidence of air fistula disease and oxygen toxicity significantly decreases, and the occurrence of hypoxic-ischemic intracranial hemorrhage is also reduced. Chronic Lung Disease (CLD), defined as the need for oxygen supply within 28 days after birth, becomes even rarer. Although there are many advantages to prevention, not all premature infants and asphyxiated infants will develop RDS. Administering PS to infants who do not develop RDS will increase costs and unnecessary endotracheal intubation. Moreover, asphyxiated and premature infants often require more urgent resuscitation, and PS prevention may temporarily interrupt the continuous process of resuscitation. Therefore, in the delivery room, for premature infants with a gestational age of less than 28 weeks or a birth weight of less than 1000g, if the mother did not receive ACH prevention before birth, PS prevention can be administered under the care of experienced and skilled resuscitation personnel. For other infants, PS should be administered immediately through mechanical ventilation and endotracheal intubation upon the occurrence of RDS, following treatment protocols.
PS prevention and PS treatment are not easily separated. Many newborns who have just been resuscitated may have irregular breathing or distress, requiring continued PS treatment. The preventive dose is similar to the treatment dose. For example, if using natural PS (whether porcine or bovine PS), the dose is 100-150mg/kg. If using synthetic Exosurf, the instillation dose is 5ml/kg (containing DPPC 67mg/kg). Refer to the treatment of respiratory distress syndrome and the overview of pulmonary surfactants and their clinical applications in Chapter 3, Section 3.
III. Combined Prevention
This refers to the combined prevention of using ACH for pregnant women before delivery and PS for newborns after delivery. It is used in the following situations: ① when prenatal prevention starts relatively late, and the pregnant woman gives birth within 24 hours, ② when newborns with severe intrauterine distress often develop severe RDS after birth, and combined prevention is advisable. Animal experiments have shown that combined prevention is more effective than single prevention.
The complications of pulmonary hyaline membrane disease often occur during oxygen therapy or in the stage of convalescence after treatment.
1. Pneumomediastinum and Pneumothorax
Due to injury of the alveolar walls, gas escapes into the pulmonary interstitium, or due to excessively high peak inspiratory pressure or mean airway pressure (MAP) during mechanical ventilation, causing interstitial pulmonary emphysema. The gas travels along the blood vessels to the mediastinum, leading to pneumomediastinum. Interstitial emphysema can also cause pneumothorax, making breathing even more difficult in cases of pneumomediastinum.
2. Oxygen Toxicity
When the inhaled oxygen concentration (FiO2) is too high or the duration of oxygen supply is too long, oxygen toxicity may occur. The most common manifestations are bronchopulmonary dysplasia (BPD) and retrolental fibroplasia. The former is a pulmonary lesion that makes it difficult to wean off the ventilator, while the latter is characterized by retrolental membrane proliferation or retinal detachment, leading to vision loss or even blindness.
3. Patent Ductus Arteriosus in the Stage of Convalescence
After mechanical ventilation and oxygen therapy, approximately 30% of cases in the stage of convalescence develop a patent ductus arteriosus. In premature infants, the tissue of the ductus arteriosus is immature and cannot close spontaneously. However, in the early stages of pulmonary hyaline membrane disease, increased pulmonary vascular resistance prevents left-to-right shunting and may even cause right-to-left shunting. In the stage of convalescence, as pulmonary vascular resistance decreases, left-to-right shunting may occur, leading to increased pulmonary blood flow, pulmonary edema, intermittent apnea, and congestive heart failure, which can be life-threatening. A systolic murmur can be heard at the left sternal border, loudest between the 2nd and 3rd intercostal spaces. If the pulmonary vascular resistance drops significantly, a continuous murmur may even be heard. Chest X-rays show an enlarged cardiac shadow and pulmonary congestion, while B-type echocardiography can directly detect the patent ductus arteriosus.
1. Group B β-hemolytic streptococcus infection
Pneumonia or sepsis caused by Group B hemolytic streptococcus infection during intrauterine or delivery processes closely resembles hyaline membrane disease and is difficult to distinguish. If the pregnant woman has a history of premature rupture of membranes or infection in the late stage [third stage] of pregnancy, the possibility of Group B β-hemolytic streptococcus infection in the infant should be considered. Blood should be promptly drawn for culture to differentiate. Before a definitive diagnosis is made, it should be treated as an infectious disease with penicillin.
2. Wet lung
Wet lung is more common in full-term infants, with mild symptoms and a short course, making it difficult to distinguish from mild hyaline membrane disease. However, the X-ray findings of wet lung are different and can be used for differentiation.
3. Intracranial hemorrhage
Intracranial hemorrhage caused by hypoxia is more common in premature infants, presenting with respiratory depression and irregular breathing, accompanied by apnea. On the other hand, hypoxia following NRDS can also cause intracranial hemorrhage. Intracranial B-ultrasound can diagnose intracranial hemorrhage.
4. Injury to the phrenic nerve
Injury to the phrenic nerve (or abnormal diaphragmatic movement) and diaphragmatic hernia can both cause respiratory distress, but cardiac and pulmonary signs and X-ray findings can be used for differentiation.





