| disease | Facial Muscle Spasm |
| alias | Facial Muscle Spasm, Hemifacial Spasm |
Facial muscle spasm, also known as facial spasm or hemifacial spasm, is a functional disorder syndrome caused by irritation of the facial nerve on one side. It mostly occurs unilaterally, with bilateral cases being rare, accounting for about 4%. The majority of patients are adults over 40 years old, with a male-to-female ratio of 2:3. The incidence rate is approximately 64 per 100,000 in the population. Stocks once reported a family with four generations where 13 individuals had philtrum involvement, but it is not considered a hereditary disease. This condition was documented as early as the 16th century in the Chinese medical text Shenshi Yaohan. However, due to the unclear disease cause and pathology, it was long regarded as an incurable condition. Otology rarely discussed this disease until the past 20 years, when extensive otoneurosurgical work led to in-depth research, establishing it as an important disease in otology.
bubble_chart Etiology
This disease can be classified into idiopathic and secondary types based on the disease cause. The secondary type is also known as symptomatic hemifacial spasm, which can be triggered by any compression or stimulation along the entire pathway from the cerebral cortex to the distal branches of the facial nerve, including sexually transmitted diseases. Common clinical causes include middle ear mastoid inflammation and tumors, space-occupying lesions in the cerebellopontine angle (such as sebaceous cysts and acoustic neuromas), as well as encephalitis, arachnoiditis, multiple sclerosis, Paget's disease, and cranial depression syndrome. Cases without identifiable causes are collectively referred to as idiopathic spasms, accounting for about two-thirds of the total cases. In 1966, Jannetta proposed that compression of the facial nerve at the root exit zone (REZ) by small stirred pulses is the primary cause of hemifacial spasm and successfully treated it with microvascular decompression, achieving satisfactory results.
bubble_chart Pathological ChangesJannetta proposed that the compression of the facial nerve at the root exit zone (REZ) by passing pulsatile vessels is the primary cause of hemifacial spasm. These vessels include the anterior inferior cerebellar artery and other pulsatile vessels, as well as tortuous, enlarged veins. As people reach middle age, these normally crossing and compressing vessels begin to harden, and increased blood pressure leads to prolonged nerve compression, which can cause demyelination. This results in abnormal electrical cross-talk between nerve axons, converting efferent signals into afferent ones. The accumulation and discharge of excessive abnormal electrical potentials can trigger hemifacial spasm episodes. This theory can also explain the pathogenesis of trigeminal neuralgia and glossopharyngeal neuralgia. However, in recent years, many scholars have disputed this view, as numerous individuals with vascular compression of the facial nerve do not develop hemifacial spasm, and 20-30% of hemifacial spasm patients show no evidence of vascular nerve compression. In recent studies, the authors measured trace elements in the serum and cerebrospinal fluid of 30 patients and found that all patients exhibited significantly reduced levels of calcium and magnesium ions. This suggests that vascular compression-induced nerve demyelination can only provoke symptoms in an environment where calcium and magnesium ions are deficient.
bubble_chart Clinical Manifestations
The twitching starts on one eyelid and gradually spreads downward to half of the facial muscles. In severe cases, it may even affect the neck and shoulder muscles. These involuntary spasms cannot be controlled by the individual and may be triggered or worsened by emotional stress or excessive fatigue. Tests indicate that these facial muscle spasms discharge synchronously at a rate of 350 times per second, manifesting as tightly closed eyelids and a twisted mouth. A single spasm can last from a few seconds to several minutes, with varying intervals between episodes. During an attack, patients may feel agitated, experience blurred vision, and occasionally suffer from facial soreness, stuffy nose, and headaches. Typically, the spasms do not occur during sleep, but about 11% of patients continue to experience them while sleeping, disrupting rest. The episodes become increasingly frequent, severely impacting daily life and work. Over time, muscle strength gradually weakens, and in the advanced stage, it may progress to a permanent deviation of the mouth on one side.
The typical spasm state, without other positive neurological signs, is generally not difficult to diagnose. Routine electroencephalography (EEG) and electromyography (EMG) should be performed. If necessary, mastoid and skull X-rays, as well as cranial CT and MRI scans, should also be conducted to rule out mastoid and skull disorders. A characteristic feature is that electrical stimulation of the supraorbital nerve on the affected side causes synchronous contraction of the orbicularis oculi muscle and other facial nerve-innervated muscles on the same side. In normal individuals or other diseases, stimulation of the unilateral supraorbital nerve only induces contraction of the orbicularis oculi muscle innervated by that supraorbital nerve.
bubble_chart Treatment Measures
(1) Drug Therapy Apart from phenytoin sodium or carbamazepine, which may be effective for some mild cases, general central nervous system sedatives, inhibitors, and hormones usually show no significant therapeutic effects. In the past, injections of procaine, absolute alcohol, or 5% phenol glycerol at the stylomastoid foramen were commonly used to induce temporary nerve fiber necrosis and degeneration, thereby reducing the conduction of abnormal excitation. A single injection dose ranged from 0.3 to 0.5 ml, with the goal of achieving grade I deviation of mouth. An excessive dose would result in permanent deviation of mouth, while an insufficient dose would lead to recurrence within 3 to 5 months. This method is now rarely employed.
Injection method: The patient lies on their side, and the area around the mastoid process on the affected side is routinely disinfected with iodine and alcohol. A 20–21 gauge needle attached to a 2 ml syringe is inserted at the junction of the cartilage at the base of the external auditory canal and the anterior edge of the mastoid process. The needle is directed forward, upward, and inward at a 30-degree angle to the horizontal line of the skull base. Upon advancing 3 cm, the needle enters a depression. First, 1 ml of 1% procaine is injected without removing the needle. After observing for 1–2 minutes, if deviation of mouth occurs, it indicates the nerve trunk has been punctured. Then, 0.3–0.5 ml of absolute alcohol or phenol glycerol is injected using the same syringe. This will result in significant deviation of mouth while the spasm disappears. After six months, the deviation of mouth often gradually resolves, but spasms recur in about two-thirds of patients.
(3) Surgical Treatment
1. Crushing of the Facial Nerve Trunk and Branch Resection Under local anesthesia, an incision is made below the stylomastoid foramen to expose the main nerve trunk. A vascular clamp is used to crush the nerve trunk, with the pressure carefully controlled. Insufficient pressure leads to short-term recurrence, while excessive pressure causes permanent deviation of mouth. Alternatively, the distal branches can be identified, and under electrical stimulation, the specific branches responsible for spasms can be selectively resected. Although this method yields better results than crushing, postoperative grade I deviation of mouth still occurs, and recurrence may happen within 1–2 years.
2. Facial Nerve Decompression This involves grinding open the bony canal through which the facial nerve exits the skull, a technique first introduced by Proud in 1953. Under local anesthesia, the mastoid is chiseled open, and an electric drill is used to completely remove the bony canal covering the horizontal and vertical segments of the facial nerve. The nerve sheath membrane is longitudinally incised to decompress the nerve fibers. In 1972, Pulec suggested that decompression limited to the mastoid was insufficient and proposed additionally grinding open the roof of the internal auditory canal and the labyrinthine segment. During surgery, pathological changes such as nerve edema, diffuse thickening, and fibrous contraction of the nerve sheath—contradictory to the disease cause—have been observed. Nevertheless, some patients have indeed been cured postoperatively. In 1965, Cawthorne reported 13 cases where no abnormalities were found. Decompression surgery is complex, and full-segment decompression is particularly challenging and carries certain risks. Whether the therapeutic effect is due to surgical trauma to the facial nerve rather than decompression remains debatable.
3. Vertical Segment Combing of the Facial Nerve Scoville (1965) used the above method to grind open the bony canal of the vertical segment of the facial nerve, then longitudinally split the vertical segment by 1 cm with a fine knife and inserted a thin silicone membrane between the split sections. The goal was to sever crossing nerve fibers to reduce abnormal impulse conduction. However, it is difficult to achieve the ideal balance where neither significant deviation of mouth nor spasms occur.
4. Facial Nerve Steel Wire Ligation This technique was designed by the author, using a 1mm diameter steel wire to ligate the facial nerve trunk for permanent crushing. The degree of ligation can be adjusted as desired. The method is simple, reliable, and suitable for elderly or frail patients who are not candidates for craniotomy exploration. It is particularly applicable to general primary healthcare facilities.
Local anesthesia is administered at the earlobe to be decocted later. A curved incision is made along the mandibular angle, and the posterior edge of the parotid gland is dissected to identify the main trunk of the facial nerve. A stainless steel wire is threaded through the periosteum anterior to the mastoid process, twisted tightly, and fixed as a fulcrum. The wire is then looped around the nerve trunk and tightened while observing facial muscle activity until the spasm ceases and a grade I deviation of the mouth is achieved. Generally, a 1–2 mm gap in eyelid closure is considered appropriate. The wire is left protruding from the incision and not cut immediately. The next morning, the recurrence of spasm is observed, and a final pressure adjustment is made before trimming the excess wire and embedding it subcutaneously. If recurrence occurs postoperatively, the incision can be reopened to locate the wire tail for further tightening. If long-term deviation of the mouth persists without recovery, the wire can also be loosened. The drawback of this method is the inevitable 3–6 months of mouth deviation postoperatively, with a relatively high recurrence rate of 30%.
5. Intracranial Microvascular Decompression Proposed by Jannetta in 1966, this is currently a commonly used method in neurosurgery internationally.
Under general anesthesia, a suboccipital or retrosigmoid approach is used. The occipital bone is removed to create a 3×4 cm bone window, and the cerebral membrane is incised to access the cerebellopontine angle. The VII and VIII cranial nerves are identified. If space-occupying sexually transmitted disease lesions or arachnoid membrane adhesions are found, they are excised and dissected. If compressive vessels are present, they are separated under microscopic diarrhea using microsurgical instruments. If separation is impossible, silicone or Teflon sheets may be interposed, or muscle fragments may be packed between the vessel and nerve. These vessels are often the anterior inferior cerebellar stirred pulse loops, which are major blood suppliers to the brainstem. Traumatic hemorrhage, induced vascular spasm, or thrombosis during surgery can lead to brainstem ischemic edema, causing severe adverse outcomes. Even spasm or thrombosis of the internal auditory stirred pulse can result in complete deafness. Clinically, it has been observed that in one-third of patients, the stirred pulse traverses between the VII and VIII nerves, or the loop apex has internal auditory stirred pulse branches entering the internal auditory canal, making vascular decompression challenging or impossible. Additionally, many patients show no identifiable compressive vessels, rendering vascular decompression inapplicable. Therefore, the author has designed a new surgical method called intracranial facial nerve trunk combing, achieving satisfactory results.
6. Intracranial Facial Nerve Trunk Combing (Nerve Longitudinal Splitting) Following the vascular decompression procedure, the cerebellopontine angle is accessed, and the VII and VIII cranial nerves are identified. The facial nerve trunk is freed, and between the brainstem root and the internal auditory canal opening, a fiber knife is used to split the nerve longitudinally into multiple layers. The number of splits is determined by the severity of the spasm, typically 10–20 times, or even dozens for severe cases. After combing the originally compressive vessels, they are restored to their original position. Follow-up over 2–5 years shows the surgical efficacy rate increased to over 98%, with recurrence reduced to 6%. The main advantages of this method are broader indications than vascular decompression, lower recurrence, and higher cure rates, particularly in reducing deafness complications. It has now replaced vascular decompression (Figure 1). Its effectiveness may stem from the disruption of abnormal potential accumulation in the nerve root zone after combing, preventing abnormal impulse discharges.
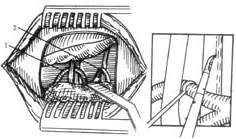
Figure 1: Intracranial Facial Nerve Combing
(1) Exposure of the cerebellopontine angle. (2) The stirred pulse is depressed, and a sharp knife is used to longitudinally comb the facial nerve. 1. Anterior inferior cerebellar stirred pulse traversing between the 7th and 8th cranial nerves. 2. Cerebellar to be decocted later stirred pulse and the glossopharyngeal nerve.
In summary, there are many surgical methods for treating facial spasm, each with its own advantages and disadvantages. Clinically, the choice should be made flexibly based on the patient's condition and medical resources. Idiopathic facial spasm is more common in adults over 40 years old and may be related to stirred pulse sclerosis and hypertensive lesions. If the patient is a young person under 30 years old, it often suggests the presence of nerve-stimulating sexually transmitted disease changes in the cerebellopontine angle, internal auditory canal, geniculate ganglion, middle ear mastoid, or parotid gland, such as congenital sebaceous cysts, hemangiomas, acoustic neuromas, and arachnoid membrane cysts. Spasm is a dangerous signal of this disease. In such cases, a comprehensive neurological examination should be conducted promptly, and cranial CT or MRI scans should be performed if necessary. Observation and waiting should be absolutely avoided to prevent delays in treatment.
Clinically, it should be differentiated from the following diseases:
1. Sequelae of facial muscle spasm after deviation of mouth: There is a clear history of deviation of mouth, caused by disordered axonal regeneration due to incomplete recovery from deviation of mouth. The affected side often exhibits varying degrees of facial muscle weakness and paralysis.
2. Idiopathic blepharospasm: This involves bilateral eyelid muscle spasms, often accompanied by mental disorders. Electromyography shows asynchronous discharges of facial muscles with normal frequency, possibly due to dysfunction of the pyramidal system.
3. Facial myokymia: This manifests as fine tremors of individual muscle bundles in the facial muscles, often spreading to surrounding eyelid muscles, usually limited to one side and may resolve spontaneously. It may be caused by benign sexually transmitted disease affecting the brainstem or cranial nerves.
4. Habitual spasm: This presents as minor spasms, purposeless stereotyped or repetitive twitching of facial muscles, mostly unilateral and often occurring in childhood.
5. Athetosis and chorea caused by midbrain and pyramidal system lesions.






