| disease | Pediatric Tuberculosis |
The incidence of subcutaneous nodules has significantly declined worldwide over the past two decades. However, in the past 10 years, the incidence has been rising year by year in regions with high rates of Acquired Immune Deficiency Syndrome (AIDS), such as North America and Africa, showing signs of resurgence and attracting widespread attention. Additionally, subcutaneous nodules remain a common disease in developing countries. It is estimated that globally, there are approximately 3 to 4 million new cases of sputum-positive (infectious) and 3 to 4 million sputum-negative (non-infectious) subcutaneous nodules each year, with about 2 to 3 million deaths annually. Three-quarters of these cases occur in developing countries, where infectious patients can account for 0.2% to 1.0% of the total population. Among children, an estimated 1.3 million new cases and 500,000 deaths occur globally each year. Developing countries report around 10 million new cases of subcutaneous nodules annually, including nearly 4 million open sexually transmitted disease cases, with approximately 3 million deaths from subcutaneous nodules. In China, the incidence and mortality of subcutaneous nodules have significantly decreased over the past 40 years.
bubble_chart Etiology
1. Morphology of subcutaneous node bacillus The subcutaneous node bacillus is slender, slightly curved, with blunt rounded ends, and often arranged in a branching pattern. It measures approximately 2–4 μm in length and 0.2–0.5 μm in width. Under an electron microscope, the outermost layer of the bacillus is the cell membrane, followed by the cytoplasmic membrane, which encloses the cytoplasm containing numerous granules, likely mitochondrial-like substances. When stained with aniline dyes, the subcutaneous node bacillus resists decolorization by acidic decolorizers, hence it is also called an acid-fast bacillus.
2. Growth characteristics of subcutaneous node bacillus The subcutaneous node bacillus grows slowly, with a reproductive division cycle of approximately 14–22 hours. Its primary nutritional requirements include glycerol, asparagine or glutamic acid, and inorganic salts such as phosphorus, potassium, sulfur, magnesium, and trace amounts of iron. It is an aerobic bacterium, with the optimal growth environment at pH 7.4 and a PO2 of 13.3–18.7 kPa (100–140 mmHg). When the pH is unsuitable or PO2 is low, such as in closed lesions or within macrophages, the metabolism of the subcutaneous node bacillus becomes inactive, leading to slow or stagnant growth and reproduction. However, it is also less susceptible to being killed by anti-tuberculosis drugs, becoming a potential source of future relapse.3. Typing of subcutaneous node bacillus The subcutaneous node bacillus can be classified into four types: human, bovine, avian, and murine. The human type is the primary pathogenic strain for humans, followed by the bovine type, while avian infections are rare and have not been reported domestically. Bovine subcutaneous node bacillus infections mainly occur due to poor management and inadequate sterilization of milk, resulting from consuming dairy products from infected cows, though such cases are now uncommon.
4. Resistance of subcutaneous node bacillus The subcutaneous node bacillus exhibits strong resistance and can survive for up to six months in dark, damp indoor environments. It dies after 2 hours of direct sunlight exposure or 10–20 minutes of ultraviolet (UV) irradiation. When using UV light, the irradiation time should be determined based on the coverage area and distance. For example, at a distance of 1 m and an area of 1 m2
, 20 minutes of irradiation can kill the subcutaneous node bacillus. It is highly resistant to acids, alkalis, and alcohol, but damp-heat has a stronger bactericidal effect. It can be killed at 65°C for 30 minutes, 70°C for 10 minutes, 80°C for 5 minutes, or boiling for 1 minute. Dry heat at 100°C requires over 20 minutes to kill it, so dry heat sterilization demands higher temperatures and longer durations. Generally, disinfection of sputum containing subcutaneous node bacillus requires extended time because the mucoprotein in sputum forms a protective layer around the bacillus, making it harder for radiation and disinfectants to penetrate. Therefore, sputum disinfection with 5% phenol or 20% bleach requires 24 hours of treatment, while 70% alcohol can kill the subcutaneous node bacillus upon contact for 2 minutes.5. Drug resistance of subcutaneous node bacillus Long-term use of subcutaneous node drugs is necessary, but irregular usage, monotherapy, or insufficient doses can easily lead to the emergence of drug-resistant strains. Experimental and clinical evidence shows that combination therapy can delay or reduce the occurrence of drug resistance.
6. Transmission routes
(1) Respiratory transmission
(2) Digestive transmission
(3) Other transmissions, such as skin transmission, placental transmission, or amniotic fluid inhalation
7. Atypical mycobacteria The bacteriological and biological characteristics of atypical acid-fast bacilli differ from those of subcutaneous node bacillus. They grow slightly faster, often produce pigments, and are non-pathogenic to guinea pigs but pathogenic to humans. Their clinical manifestations resemble subcutaneous node disease, but they exhibit resistance to subcutaneous node drugs. Atypical acid-fast bacilli cause peripheral lymphadenitis in children, particularly cervical lymphadenitis. After infection with atypical mycobacteria, the tuberculin PPD-S (standard purified protein derivative) elicits a weak reaction, while homologous antigens such as PPD-F, PPD-Y, PPD-B, and PPD-G (atypical mycobacterial antigens) produce strong positive reactions. In regions with low tuberculin sensitivity, the possibility of atypical mycobacterial infection should be considered.
bubble_chart PathogenesisWhen droplet nuclei containing subcutaneous node bacilli are inhaled into the human body, they settle in the marginal areas of the pulmonary lobules beneath the pleural membrane, mostly in the lower two-thirds of the lungs. If the body's resistance is weak, primary pulmonary subcutaneous node disease may develop. Although the subcutaneous node bacilli can be engulfed by alveolar macrophages, due to low cellular immunity, most of them cannot be killed, allowing the subcutaneous node bacilli to multiply within the cells and potentially be carried by macrophages to various parts of the body. Since the specific immune response to subcutaneous node antigens develops slowly, delayed hypersensitivity occurs approximately 3 to 6 weeks later, forming epithelioid cell nodules, granulomas, and surrounding exudative reactions in the lungs, which constitute the primary lesion. The primary lesion is always located at the base of the upper lobe or the upper part of the lower lobe, near the pleural membrane. Simultaneously, the subcutaneous node bacilli travel along the lymphatic vessels to the hilar and mediastinal lymph nodes, causing lymphangitis and lymphadenitis, which together form the three components of the primary complex. When the number of subcutaneous node bacilli is large and their virulence is strong, the body enters a state of high hypersensitivity, and the exudative inflammation around the lesion can expand to most pulmonary segments or even an entire lobe. Meanwhile, due to tumor necrosis factor released by activated macrophages, tissue destruction and caseous necrosis occur in the primary lesion and subcutaneous node granulomas within the lymph nodes. Caseous lesions in the hilar or even lymph nodes may rupture into the bronchi, leading to bronchial dissemination, or spread hematogenously to cause blood-borne dissemination.
bubble_chart Pathological Changes
After the bacillus invades the human body, it causes specific and non-specific tissue reactions in the tissues. The basic tissue changes in subcutaneous node inflammation are exudation, proliferation, and degeneration. In exudative changes, the exudate consists of inflammatory cells, serous fluid, and fibrin, with monocytes and fibrin being the main components. Proliferative changes are primarily characterized by subcutaneous node granulomas, followed by exudation and degeneration. The formation of epithelioid cell nodules and the presence of Langhans giant cells are the main features of subcutaneous node inflammation. The characteristic change in the degenerative process is caseous necrosis, which often occurs in exudative sexually transmitted disease changes. The favorable outcomes of subcutaneous node inflammation are absorption, fibrosis, calcification, and ossification.
1. Primary Complex After the pathogen enters the lungs through the respiratory tract, it produces the initial infection focus in the alveoli, mostly located in the lower part of the upper lobe of the lung, especially on the right side, near the pleura. There is usually one focus, occasionally two or more. The primary focus initially manifests as desquamative pneumonia or fibrinous pneumonia, with central caseous necrosis, followed by proliferative subcutaneous node nodules appearing around it. Subsequently, a fibrous capsule forms around the focus. The pathogen invades the hilar lymph nodes via the surrounding lymphatic vessels. Caseous lymph nodes often adhere to each other and cling to the bronchial wall. In summary, the primary complex consists of four components (Figure 1): ① The initial pulmonary infection focus; ② Bronchial lymph node subcutaneous node; ③ Lymphangitis connecting the initial focus to the lymph nodes; ④ Pleuritis adjacent to the initial focus. The primary complex is not only seen in the lungs but can also occur in the intestines, pharynx, skin, etc.
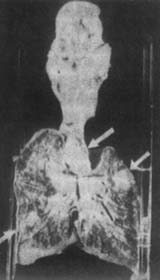
Figure 1 Primary Complex
1 year and 3 months, female, primary complex with hematogenous dissemination. The right lung section shows enlarged hilar lymph nodes, caseous necrosis foci, and scattered millet-sized gray-yellow nodules throughout, with some foci fused.
The primary complex generally tends to heal, with a fibrous capsule forming around the focus and the central caseous material eventually calcifying. Calcification as a healing method is one of the characteristics of pediatric subcutaneous node disease. However, in young patients with a large bacterial load and weak resistance, the condition may worsen, often leading to the following pulmonary complications: ① Expansion of the initial pulmonary infection focus, with central caseous necrosis forming a primary cavity, sometimes causing bronchial, lymphatic, or hematogenous dissemination. ② Bronchial lymph nodes penetrating the bronchus, leading to bronchogenic disseminated caseous pneumonia (Figures 2, 3). Bronchial obstruction may cause atelectasis or emphysema. Occasionally, lymph node foci or subcutaneous node meningitis may occur. The extensive involvement of the lymphatic system and the tendency for systemic hematogenous dissemination are characteristics of pediatric subcutaneous node disease.
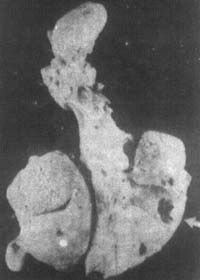
Figure 2 Right Lung Caseous Pneumonia with Cavity Formation in the Right Middle Lobe
4 months, female, caseous pneumonia with cavity formation. Enlarged hilar lymph nodes, multiple gray-yellow caseous necrosis foci on the right lung section, some fused, and subpleural cavity formation in the right middle lobe.
Gross pathological changes in subcutaneous node bronchitis include bronchial compression and deformation, rough mucosal surfaces, lymphobronchial fistulas, granulomas, and caseous material emboli in the bronchi (Figure 5).
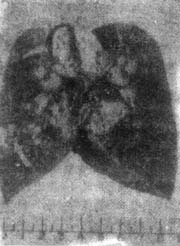
Figure 3 Lobular Caseous Pneumonia
2 months, male infant, right lung lobular caseous pneumonia. The right lung section shows scattered gray-yellow caseous necrosis foci the size of broad beans (arrows), with denser foci near the hilum. Enlarged hilar lymph nodes with caseous necrosis (arrows).
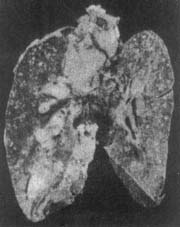
Figure 4 Foxtail millet-sized pulmonary subcutaneous node
(Pathological specimen from Shanghai Medical University Medical Hospital)
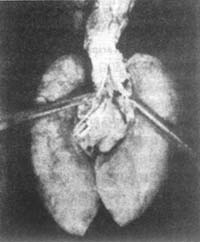
Figure 5 Bronchial fistula of lymph node
Caseous material embolus in the bronchus. Arrow indicates the perforation site
(Pathological specimen from Shanghai Medical University Medical Hospital)
Additionally, pulmonary subcutaneous nodes can cause bullous lung qi swelling and honeycomb-like lung qi swelling, which are more commonly seen in treated foxtail millet-sized pulmonary subcutaneous nodes. The occurrence of bullous lung qi swelling is due to the rapid absorption of caseous lesions after treatment, allowing air to enter the cavity and causing acute expansion. Honeycomb-like lung qi swelling is caused by incomplete valvular obstruction of the bronchus; air can enter the alveoli, but exhalation is difficult, leading to gas retention. At the same time, due to the reduced elasticity of the affected alveolar walls, honeycomb-like lung qi swelling is more likely to form.
2. Secondary pulmonary subcutaneous node Also known as adult-type subcutaneous node. Pathogenesis: ① External reinfection: After the primary subcutaneous node becomes quiescent, reinfection with subcutaneous node bacilli occurs, often in the upper lobes of the lungs. The tissue reaction during reinfection is more intense. The lesions are infiltrative, surrounded by extensive exudative tissue changes. It is more common in older children or adults, hence the term adult-type subcutaneous node. ② Reactivation of internal lesions. Most of these lesions originate from old remnants of primary hematogenous dissemination, commonly found in the lung apices. They can also arise from the deterioration of previously healed primary lesions.
Compared to primary subcutaneous nodes, secondary subcutaneous nodes have the following differences: Secondary subcutaneous nodes are mostly located in the lung apices, rarely involve pulmonary lymph nodes, easily form cavities and spread along the bronchi, exhibit extensive fibrosis during the healing phase, and may even show sclerosis, but calcification is less common; infiltrative sexually transmitted disease changes are prone to caseous necrosis, forming cavities and spreading via the bronchi. Sometimes, due to the fibrous limitation of caseous pneumonia or obstruction of the draining bronchus of a cavity, spherical lesions filled with caseous material may form, naturally referred to as subcutaneous node tumors. Their diameter is generally 2–4 cm, mostly located in the upper lobes of the lungs near the pleural membrane.
bubble_chart Clinical Manifestations
(1) Subcutaneous node allergic manifestations such as herpetic conjunctivitis, erythema nodosum, scrofulous facies, subcutaneous nodular Bi disease (Poncet's arthritis), etc.
(2) Fever In acute onset cases, high fever may accompany with significant fluctuations in fever pattern, often indicating severe progressive subcutaneous nodular manifestations, such as millet-seed type subcutaneous nodules, caseous pneumonia, etc. However, most pediatric patients present with irregular low-grade fever, more pronounced in the afternoon, with daily temperature fluctuations often exceeding 1°C. The fever is evident, but systemic symptoms are relatively mild, which is characteristic of subcutaneous nodular disease.
(3) Neurological symptoms Such as lethargy, dysphoria, irritability, sleep disturbances, as well as night sweats, facial flushing, and other symptoms of autonomic dysfunction.
(4) Chronic toxic symptoms Such as loss of appetite, emaciation, fatigue, personality changes, and developmental delays.
(5) Respiratory symptoms Apart from paroxysmal cough or even dyspnea caused by enlarged lymph nodes compressing the bronchi, and corresponding symptoms and signs due to massive pleural effusion, general respiratory symptoms are usually minimal. Positive signs may be absent or very few. The inconsistency between respiratory symptoms, signs, and X-ray findings is a characteristic feature.
(6) Generalized lymphadenopathy Early subcutaneous nodular toxic symptoms include varying degrees of generalized lymphadenopathy, but the lymph nodes are soft in texture. In chronic subcutaneous nodular toxicosis, the enlarged lymph nodes become firm, which can serve as a diagnostic reference.
1. History of contact with subcutaneous nodes, especially with individuals having open sexually transmitted diseases. The contact history can also provide clues and evidence as to whether the child is infected with drug-resistant pestilence, which is significant for determining the treatment plan.
2. Recent history of acute pestilence, with measles, chickenpox, and whooping cough being the most important.
3. History of BCG vaccination. Not only should the vaccination history be inquired about, but the vaccination scar should also be examined to confirm the number of vaccinations and the method of administration.
4. See clinical symptoms.
5. Subcutaneous node (tuberculin) skin test. This is an important method for screening the infection rate of subcutaneous nodes in populations not vaccinated with BCG, as well as a powerful tool for the clinical diagnosis of subcutaneous node disease. In the past, old tuberculin (OT) was commonly used, but currently, the more accurate and stable purified protein derivative (PPD) of subcutaneous nodes is employed. An intradermal injection of 5U OT or PPD resulting in a reddened induration of 5mm or more after 72 hours is considered a positive reaction.
A negative test result indicates: ① No infection—generally, a 1mm negative OT result can exclude subcutaneous nodes, but false negatives should be noted; ② Biological healing of the lesion, which is rare in practice. A positive test is seen in: ① Post-BCG vaccination (usually a weak positive reaction); ② Natural infection without disease onset; ③ Onset of subcutaneous node disease; ④ Previous subcutaneous node disease that has healed.
Some determine different criteria for a positive tuberculin reaction based on whether the child is in a high-risk state for subcutaneous nodes or has a history of BCG vaccination. For example, children with close contact with subcutaneous nodes or those with compromised immune systems are considered high-risk, and an induration diameter of ≥5mm is deemed positive. In areas with a high incidence of subcutaneous nodes, extremely impoverished populations in any country, as well as individuals with poor nutritional status, diabetes, or chronic sexually transmitted diseases, the positive criterion is an induration diameter of ≥10mm. For the general population (excluding the above two groups) who have received BCG vaccination, an induration diameter of ≥15mm is considered a positive reaction.
Since both OT and PPD contain antigens from various mycobacteria (including subcutaneous node and non-subcutaneous node strains) as well as common antigens from BCG, cross-reactions can occur. Therefore, when determining whether an infection is due to subcutaneous nodes, positive reactions from non-subcutaneous node mycobacteria or BCG vaccination should be excluded. Given the widespread use of BCG vaccination in China, the most critical issue now is distinguishing between natural infection and post-BCG vaccination positive reactions. Generally, the former shows a stronger positive reaction, with deeper red induration, harder and thicker texture, clear edges, and a larger area (often exceeding 15mm in diameter). The induration persists for 72–96 hours and leaves pigmentation after fading. If the subcutaneous node (tuberculin) test result cannot distinguish between natural infection and post-BCG vaccination reaction, the test should be repeated after six months to one year. BCG-positive reactions tend to weaken over time, whereas natural infection reactions remain unchanged. If the tuberculin test reaction increases from below 10mm to above 10mm within the past two years, with an increase of ≥6mm, it is often indicative of new contraction after BCG vaccination (refer to Tables 15-1 to 3).
Table 15-1: Differentiation Between Natural Infection and Post-BCG Vaccination Positive Results (Part 1)
| Positive Result | Natural Infection | Post-BCG Vaccination |
| Degree of Positivity | Strong | Weak |
| Changes in Positivity | Minimal variation | Gradually weakens over time |
| Duration of Positivity | Long-lasting, potentially lifelong | Short duration, disappears after 3 to 4 years |
Table 15-2 Second Method for Differentiating Positive Results Between Natural Infection and BCG Vaccination
| Time After Vaccination | Only Mild Redness and Minimal Induration | Redness and Induration | Double-Ring Red Induration |
| Within 6 Months | Positive After BCG Vaccination | Positive After BCG Vaccination | Positive Due to Natural Infection |
| 6 Months to 1 Year | Positive After BCG Vaccination | Positive Due to Natural Infection | Positive Due to Natural Infection |
| Over 1 Year | Positive After BCG Vaccination | Positive Due to Natural Infection | Positive Due to Natural Infection |
Table 15-3 Third Method for Differentiating Positive Results Between Natural Infection and BCG Vaccination
| Color | Texture | Thickness | Margin | Area | Reaction >15mm | Strong Positive Reaction | Duration | |
| Natural Infection | Deep Red | Hard | Thick | Sharp | Large | Common | Frequent | Long, Often Lifelong, Not Easily Faded |
| BCG Reaction | Light Red | Not Hard | Thin | Unclear | Small | Rare | None | Short, Gradually Fades After 3-5 Years |
The nature of tuberculin reactions differs between BCG vaccination and natural infection. The reaction post-BCG vaccination is a protective "preventive" cell-mediated immune response, whereas the tuberculin reaction from natural infection is a Koch-type reaction. This involves a stronger hypersensitivity response caused by sensitized T-lymphocytes re-exposed to antigens, leading to macrophage release of tumor necrosis factor. Hence, positive reactions from natural infection are generally stronger.
To differentiate between subcutaneous node and non-subcutaneous node mycobacterial infections, specific antigens for various mycobacteria (e.g., PPD-S for subcutaneous node bacteria and PPD-B, -F, -G, -Y for non-subcutaneous node mycobacteria) are often used in skin tests for identification.
6. X-ray Examination Since over 95% of infections occur via the lungs, X-ray examination is crucial, especially for children with positive tuberculin test results.
(1) Chest fluoroscopy can be performed with multi-axis透视 to achieve the following purposes: to clarify whether there are enlarged lymph nodes or nodular shadows in the hilar region that cannot be dispersed by rotation. During the examination, attention should be paid to whether there are lesions in the ribs, mediastinum, and behind the heart, and the location of the lesions should be determined, including which肺叶 or肺段 is affected. The condition of the organs should be thoroughly examined, and the relationship between mediastinal摆动 and respiration should be noted. For example, if there is a blockage in the right bronchus, air cannot smoothly enter the right lung during inhalation, causing more air to enter the left lung and increasing pressure in the left lung, resulting in the mediastinum摆动 to the right side.
(2) Frontal view Observe the shape of the thorax, lungs, heart, and diaphragm to determine the location of lesions. For example, to distinguish between intrapulmonary and mediastinal lesions: if the center of the lesion is in the lung, the edge of the shadow forms an acute angle with the mediastinum; when the lesion is in the mediastinum, the edge of the shadow forms an obtuse angle with the mediastinum. Similarly, to differentiate between intrapulmonary lesions and pleural lesions: if the lesion is intrapulmonary, its center is in the lung, and the edge of the shadow forms an acute angle with the chest wall; if the lesion is pleural, its center is in the pleura, and the edge of the shadow forms an obtuse angle with the chest wall.
(3) Lateral view The purpose is to determine the location of the lesion in the mediastinum (anterior, middle, or posterior mediastinum); identify which lobe or segment of the lung is affected; reveal tracheal bifurcation lymph nodes; display interlobar pleuritis of the horizontal or oblique fissures; show lesions obscured by the heart or diaphragm on the frontal view; and differentiate between atelectasis, pneumonia, and effusion.
(4) Tomography Can assess mediastinal or hilar lymph node enlargement. Evaluate whether bronchi are compressed, narrowed, obstructed, or dilated. Serves as an auxiliary examination for bronchoscopy and bronchography. Detects cavities within the lung parenchyma and the condition of draining bronchi. Checks for lesions behind the heart, diaphragm, clavicle, or thickened pleura.
(5) CT examination Due to its cross-sectional imaging, absence of structural overlap, and high resolution, CT often reveals lesions not easily detected on plain X-rays, such as those obscured by the mediastinum, diaphragm, or ribs. For example, CT can frequently identify multiple enlarged lymph nodes not visible on plain films, particularly subcarinal lymphadenopathy. A non-contrast scan is typically performed first, with contrast-enhanced scanning if necessary. Post-contrast enhancement increases density and lesion conspicuity, aiding in the detection of subcutaneous node lesions, such as early atypical miliary dissemination. The high resolution of contrast-enhanced scans reflects physical changes more reliably. Additionally, caseous necrosis in subcutaneous node lesions appears as a low-density ring shadow on contrast-enhanced CT, assisting in qualitative diagnosis. CT also has a higher detection rate for characteristic calcifications. In differential diagnosis, CT is more accurate for identifying subcutaneous node cavities, atelectasis, and bronchiectasis. Furthermore, CT can detect small pleural effusions and subcutaneous node lesions obscured by effusion. Contrast-enhanced scans also help distinguish encapsulated effusions from pulmonary infiltrates and atelectasis.
7. Laboratory tests
(1) ESR (Erythrocyte Sedimentation Rate) Changes in ESR can help assess disease activity, treatment efficacy, and prognosis. Asymptomatic primary subcutaneous node disease often shows normal ESR, but note that symptomatic cases may also occasionally have normal ESR.
(2) Subcutaneous node bacilli examination Samples may include gastric fluid, bronchial washings, cerebrospinal fluid, pleural effusion, ascites, pericardial effusion, urine, stool, or local lesion secretions (e.g., skin miliary nodules or fistula discharge) for smear or culture to identify subcutaneous node bacilli. Guinea pig inoculation may be performed if necessary, with sample selection based on clinical presentation.
(3) Protein electrophoresis Helps evaluate disease progression and recovery. α 2 and γ globulin levels often rise proportionally with disease severity, including in cases with complications. However, in tuberculous meningitis and severe miliary subcutaneous node disease in children, γ globulin may initially decrease. If treatment is ineffective, it does not rise; if treatment is successful and symptoms improve, γ globulin gradually increases to normal levels.
(4) C-reactive protein (CRP) Most cases of active pulmonary subcutaneous node test positive, while inactive cases are negative.
(5) Bronchography Evaluates bronchial morphology and spatial relationships. Its purposes include: ① Assessing bronchial changes in subcutaneous node disease, such as bronchial fistulas, stenosis, bronchiectasis, or atelectasis. ② Aiding in differential diagnosis, distinguishing pulmonary subcutaneous node from lung cysts or abscesses, and differentiating pulmonary, pleural, and mediastinal lesions.
(6) Bronchoscopy Purpose: ① To observe whether there is obstruction, displacement, stenosis, or compression in the bronchi. It can also be used for treatment, such as dilating or repositioning stenotic or compressed lumens. ② To examine the bronchial membrane for lesions, such as cyanotic swelling, ulcers, granulation, caseous masses, or secretions, for pathological examination and subcutaneous node bacterial culture. ③ To remove bronchial granulation and relieve obstruction.
(7) Chest Ultrasound Examination Ultrasound examination is helpful in differentiating pulmonary subcutaneous nodes from pulmonary cysts or abscesses, and it can also assist in the differential diagnosis of atelectasis and pleural effusion. The accuracy of ultrasound in locating pleural effusion is very high, as it can indicate the location, depth, and extent of the effusion, particularly aiding in the diagnosis and drainage of encapsulated effusions. Ultrasound can detect small amounts of pleural effusion, especially when accompanied by pleural thickening that is difficult to assess on plain X-rays. The detection of hypoechoic areas by ultrasound is beneficial for diagnosis and treatment. Additionally, ultrasound has quantitative, qualitative, and locational significance for pericardial effusion, encapsulation, adhesions, thickening, and constriction, being far more sensitive than plain X-rays and CT scans, and thus holds significant diagnostic value.
8. Biopsy As an invasive procedure, biopsy requires careful execution: ① Peripheral lymph node aspiration or biopsy: Before forming lesions, subcutaneous nodes go through a bacteremia phase, which can lead to lesions in bones, the liver, spleen, lymph nodes, etc. Sometimes, lymph node lesions become apparent before pulmonary lesions develop. Lymph node biopsy may reveal typical subcutaneous node nodules, caseous necrotic tissue, and Langhans giant cells. ② Pleural biopsy: When it is difficult to distinguish between subcutaneous node and non-subcutaneous node pleural effusion, pleural biopsy can aid in diagnosis. Subcutaneous node cases may show subcutaneous node nodules, but a negative result does not completely rule out the condition, limiting its practical utility. ③ Lung biopsy: For undiagnosed pulmonary lesions, lung biopsy with bacterial culture can clarify the pathogen. Due to its invasive nature, this method is rarely used clinically.
9. New Diagnostic Methods for Subcutaneous Nodes
(1) Serological Diagnosis Detection of antibodies in blood, sputum, or cerebrospinal fluid. Currently, three types of antigens are used:
① Crude subcutaneous node bacterial antigens: These include antigens from subcutaneous node bacterial culture filtrates, saline-extracted subcutaneous node antigens, polymerized tuberculin antigens, and antigens derived from ultrasonically treated BCG.
② PPD antigens: Such as PPD-S, PPD prepared from BCG, and PPD-B prepared from non-subcutaneous node mycobacteria.
③ Purified subcutaneous node bacterial antigens: Examples include subcutaneous node bacterial antigens 5 and 6; P32 (Purified 32KDa), a 32KDa protein antigen purified from BCG culture filtrates in 1987; mycobacterial glycolipids such as SAGA1B1 and C purified from BCG; and purified antigens isolated using immunoaffinity columns prepared with TB-C-1 antibodies.
Methods for antibody detection include ELISA and ABCELISA.
(2) Detection of Subcutaneous Node Bacterial Components Techniques such as gas chromatography-mass spectrometry with selected ion monitoring, negative-ion mass spectrometry, and frequency-pulsed electron capture gas-liquid chromatography are used to detect subcutaneous node stearic acid in sputum, serum, and CSF for diagnosing pulmonary subcutaneous nodes and subcutaneous node meningitis. These tests are highly sensitive but require complex equipment and expertise, making clinical adoption challenging at present.
(3) DNA Probes DNA probes are used to detect specific ribosomal RNA sequences of particular mycobacterial species (e.g., subcutaneous node bacteria). Isotope-labeled DNA is added to samples containing subcutaneous node bacterial RNA for hybridization. Indirect probes are currently available on the market.
(4) PCR (polymerase chain reaction) polymerase chain reaction - Uses exogenous gene amplification to detect extremely small amounts of subcutaneous node bacterial gene fragments in cerebrospinal fluid or other specimens for early and rapid diagnosis of subcutaneous node disease.
bubble_chart Treatment Measures
1. Early treatment: In the early stages of the disease, the bacteria are in a state of growth and reproduction, with active metabolism, making it easier for drugs to take effect, and the early lesions are more easily repaired.
2. Appropriate dose: The dose should be sufficient to achieve maximum bactericidal or bacteriostatic effects while remaining tolerable to the patient, with minimal toxic reactions. If the dose is insufficient, not only will the treatment be ineffective, but it may also lead to the development of drug resistance.
3. Combination therapy: This is because: ① Different bacteria within a population vary in their sensitivity to drugs, and there are naturally occurring resistant variants in varying proportions. Combination therapy can prevent the emergence of resistance. ② Combination therapy can target bacteria in various metabolic states, both intracellular and extracellular, to enhance therapeutic efficacy. When combining drugs, those with synergistic effects should be selected, such as INH combined with RFP or PAS, or RFP combined with EB. However, certain drugs should not be combined under the following circumstances: ① Those with the same side effects; ② Those with cross-resistance; ③ Those with antagonistic effects; ④ Those that are too weak in efficacy.
4. Regular medication: Medication should not be interrupted arbitrarily, as this may lead to the development of resistant strains. As for intermittent therapy, there are specific requirements regarding dose and intervals, and its usage follows certain rules, which does not constitute arbitrary interruption.
5. Full-course adherence: To eliminate persistent bacteria and prevent relapse, chemotherapy must be adhered to for the full course. In recent years, short-course chemotherapy has emerged, but whether the course is shortened to 9 months or 6 months, full-course adherence is still essential.
6. Phased treatment: Whether it is the traditional long-course therapy or the newer short-course chemotherapy, treatment should be divided into stages: ① Intensive phase: Strong drugs are used in combination to rapidly eliminate sensitive bacteria and those in active growth and division, while effectively addressing any existing resistant bacteria. In traditional chemotherapy, the intensive phase typically lasts six months, whereas in short-course chemotherapy, it lasts 2–3 months. This is the critical phase of chemotherapy. ② Consolidation (continuation) phase: The goal is to clear persistent bacteria, consolidate treatment outcomes, and prevent relapse. Traditional chemotherapy generally lasts six months or even four months.
Table 15-5: A brief list of currently used anti-subcutaneous node drugs
| Drug name | Daily dose | Toxicity | Allergic reactions | Precautions | |
| Adults | Children | ||||
| Isoniazid (INH) | 5–8 mg/kg, usually 300 mg | 10–20 mg/kg, not exceeding 400 mg | Peripheral neuritis with sensory abnormalities, liver function impairment (rare in children, more common in adults), convulsions (rare), optic neuritis and atrophy (rare), others (muscle tremor, dizziness, lethargy, euphoria, mental excitement or disturbance, gastrointestinal disorders, low fever; arthralgia, myalgia, nausea, poor appetite, breast enlargement, dysuria) | Fever, rash, increased eosinophils in blood | Administer vitamin B6 to adults with a dosage exceeding 5mg/kg, and it should also be given to other indicated individuals. Rifampin may enhance the hepatotoxic effect of INH, and the dosage of both should be appropriately reduced when used together, preferably not exceeding mg/kg. Liver function tests should be performed monthly. |
| Streptomycin sulfate streptomycin (SM) | 15mg/kg, usually 1g initially, then 1g, 2-3 times per week | 20-30mg/kg, not exceeding 0.75g | Damage to the 8th cranial nerve such as vestibular dysfunction causing vertigo, nausea, ataxia, hearing impairment like tinnitus and high-frequency hearing loss; renal damage (rare), peripheral neuritis (rare); perioral numbness, pain at injection site, tightness in the head, difficulty concentrating, vision impairment | Rash, fever, lymphadenopathy (rare), eosinophilia, anaphylactic shock reaction (rare) | Routine hearing tests for elderly patients, hematuria and blood urea nitrogen tests; others may have vestibular and auditory functions checked as appropriate, careful observation is required |
| Sodium para-aminosalicylate para-amino- salicylic acid (PAS) | 150-200mg/kg, usually 9-12g | 150-200mg/kg, not exceeding 8g | Gastrointestinal symptoms (anorexia, nausea, abdominal distension and fullness, diarrhea), liver function impairment, goiter and hypothyroidism, coagulation disorders, bone marrow suppression, anemia (in those with glucose-6-phosphate dehydrogenase deficiency), leukopenia, proteinuria | Rash, fever, lymphadenopathy, myalgia and arthralgia, angioneurotic edema, purpura, eosinophilia, mononucleosis-like syndrome | Take with meals, can be taken with antacids, start with small doses and gradually increase to appropriate amount, side effects often occur 3-5 weeks after treatment, check liver function monthly |
| Rifampicin (Rifamycin) Rifamicin(RFP) | 450-600mg once daily, taken orally 1 hour before breakfast | 10-mg/kg | Liver function impairment (elevated GPT, bilirubin, cholesterol, triglycerides), abdominal distension and fullness and loss of appetite and other gastrointestinal symptoms, blood cell reduction, thrombocytopenia, anemia, elevated blood urea nitrogen, neurological symptoms such as headache, drowsiness, vision impairment, ataxia, confusion, etc. More common with intermittent use than daily use | Rash, fever, flu-like symptoms, eosinophilia, anaphylactic shock (rare, seen occasionally with intermittent dosing) | Liver function impairment is more likely when combined with INH, mostly occurring within the first 2 months of treatment, check liver function monthly |
| Ethambutol ethambutol(EB) | 15mg/kg, not exceeding 1.0g | 15-25mg/kg | Retrobulbar neuritis, with reduced visual acuity, visual field abnormalities, central scotoma and reduced green color perception, visual field loss (higher incidence with larger doses), peripheral neuritis, headache, gastrointestinal reactions (such as nausea, vomiting, diarrhea), occasional liver function impairment | Rash | Check visual acuity, visual fields and color discrimination monthly, those with poor vision should have fundus examination and follow-up changes |
| Ethionamide alpha-ethye- thioisonicoti -namide(1314TH) Ethionamide prothicamide(1321TH) | 10~15mg/kg, usually 1g | 10~15mg/kg | Gastrointestinal symptoms, including loss of appetite, nausea, vomiting, liver function impairment, headache, insomnia, vertigo, postural hypotension, mental depression or excitement, acne, alopecia areata, stomatitis, glossitis, angular cheilitis, peripheral neuritis (rare), spasm (rare), gynecomastia, impotence | Rash, purpura, arthralgia | Liver function should be checked monthly. Take with meals, can be taken with antacids. Vitamin B6 can prevent side effects. Nicotinamide and vitamin B complex are also needed. Concurrent use with cycloserine may lead to psychiatric side effects. Concurrent use with PAS may cause thyroid dysfunction |
| Pyrazinamide (PZA) | 30mg/kg, not exceeding 2g | 20~90mg/kg, not exceeding 1.5g | Liver function impairment, hyperuricemia, painful wind arthritis, gastrointestinal symptoms such as loss of appetite, nausea, vomiting, increased difficulty in diabetes management (rare) | Fever, rash, arthralgia | Check GOT monthly, monitor liver and spleen enlargement, timely check for hematuria acid |
| Kanamycin (KM) | 10~15mg/kg | 10~15mg/kg | Damage to the 8th cranial nerve, such as vestibular damage with vertigo, nausea, etc.; cochlear damage, manifested as high-frequency hearing loss, kidney damage (proteinuria, hematuria, cast urine, etc.), neuromuscular junction curare-like effect (mostly during intraperitoneal drip), paresthesia, dysphoria, headache, nausea, loss of appetite, long-lasting nodules and pain at the injection site | Eosinophilia (8~10%), fever, rash, itching, anaphylactic shock (rare) | Monthly hearing tests, hematuria acid check, urine examination, timely serum electrolyte check |
| Viomycin (VM) | 15mg/kg | 10mg/(kg·d) or 30mg/kg, twice weekly | Vestibular damage, vertigo, cochlear damage, hearing loss, tinnitus, kidney damage (proteinuria, cast urine, increased red blood cells, increased white blood cells), elevated hematuria nitrogen, electrolyte imbalance, hypocalcemia and hypokalemia, nausea, vomiting, fluid retention and edema, pain at the injection site | Eosinophilia, rash, anaphylactic shock (rare), high fever | Monthly hearing tests and hematuria nitrogen, serum electrolytes, urine examination. Rarely used due to high toxicity |
| Capreomycin (CP) | 15mg/kg, not exceeding 1g | 15mg/kg | Kidney damage, vestibular damage, cochlear damage, hearing loss | Eosinophilia, rash, fever, anaphylactic shock | Monthly urine check, hematuria nitrogen, and hearing tests |
| Cycloserine (CS) | 10mg/kg, not exceeding 0.5g | 10~15mg/kg | Severe cases may develop psychosis, spasm (occurring in 5~10% of patients with a daily dose of 1g), delusions, dementia, depressive states, drowsiness, muscle weakness, headache, dizziness, memory impairment, grade I speech障碍, grade I vision障碍 tremor, hyperreflexia, ankle clonus | fever, rash | Contraindicated in patients with a history of epilepsy or mental illness. Renal function should be checked before medication. For patients with renal insufficiency, drug blood concentration should be monitored. Vitamin B6 should be administered, especially when combined with INH. Side effects are dose-dependent. Vitamin B6, phenobarbital, and phenytoin sodium can control toxic reactions. |
| Thioacetazone para-acetyl- aminosemi- carbasore | 2.5mg/kg, 100~150mg, not exceeding 150mg | 1~2mg/kg, not exceeding 40mg | Liver function impairment, hematopoietic disorders such as anemia, granulocytopenia, purpura; gastrointestinal disturbances such as loss of appetite, nausea, vomiting Renal impairment such as proteinuria, etc. | Rash, eosinophilia, conjunctivitis, neuritis | Contraindicated in patients with liver or kidney diseases. More effective for lymph node subcutaneous nodes and membrane subcutaneous nodes (e.g., laryngeal subcutaneous nodes, bronchial subcutaneous nodes, and intestinal结核). Not used for subcutaneous node性脑膜炎, miliary subcutaneous nodes, or caseous pneumonia. |
Short-course chemotherapy is a new regimen that has emerged in the past decade. Its efficacy depends on two factors: (1) the drug's early bactericidal effect on rapidly growing, metabolically active subcutaneous node bacilli to prevent the emergence of drug-resistant strains; (2) the drug's sterilizing effect on slowly growing, metabolically inactive subcutaneous node bacilli (persisters) to prevent relapse. Therefore, the principles for selecting drugs in short-course chemotherapy are: ① For actively dividing and metabolically vigorous bacteria, potent bactericidal drugs such as SM, INH, and RFP should be used. ② Consider using sterilizing drugs effective against persisting subcutaneous node bacilli to prevent relapse, such as PZA, RFP, and INH. ③ Bacteriostatic drugs such as EMB, PAS, ETH, and TB1 are not suitable for short-course chemotherapy.
For pediatric short-course chemotherapy, the following points should be considered: ① Pediatric subcutaneous node disease is often a recent infection, especially hematogenous dissemination, so preventing brain membrane involvement is most critical. First, choose drugs that easily cross the brain membrane into the cerebrospinal fluid, such as INH, RFP, and ETH, but not EB. ② For acute hematogenous dissemination, it is best to use drugs that kill rapidly growing bacteria, such as SM. ③ For subcutaneous node性脑膜炎, consider using injectable anti-subcutaneous node drugs, such as SM. ④ Primary drug-resistant INH and SM subcutaneous node bacilli infections are more common in children than in adults. In addition to selecting drugs based on the治疗效果 of the pestilence source, choose potentially sensitive bactericidal drugs such as RFP and PZA or ETH and EB. ⑤ In acute subcutaneous node infections, monocytes are often affected, so emphasis should be placed on using intracellular bactericidal drugs (e.g., PZA). ⑥ The replication cycle of subcutaneous node bacilli is 14~22 hours, so medication can be administered once daily. It has been proven that the peak concentration of INH administered at draught is more important than the constant concentration of conventional multiple daily doses. Therefore, administering the full daily dose at draught not only improves efficacy but also ensures adherence to the medication regimen.
1. Strengthen Primary Healthcare: Rely on the grassroots medical networks in urban and rural areas, and fully utilize the roles of doctors at all levels, including village doctors. Clinical evidence shows that the occurrence of subcutaneous node is closely related to children's health status and living environment. Attention should be paid to proper nutrition, good hygiene habits, and preventive measures such as vaccination against measles and whooping cough.
2. Early Detection and Prevention: Early detection is a prerequisite for early treatment in children. Regular physical examinations should be conducted to identify diseases early. Children in contact with active pulmonary subcutaneous node patients have significantly higher infection, morbidity, and prevalence rates compared to the general population.
3. Conduct Education and Emphasize Isolation: Carry out extensive health education to ensure the public has a correct understanding of subcutaneous node disease. Implement proper disinfection and isolation measures in households with subcutaneous node patients to protect children from pestilence. Additionally, other preventive measures should be noted, such as dairy cow management, milk sterilization, premarital and prenatal check-ups, and promoting the habit of not spitting indiscriminately.
4. BCG Vaccination:
(1) **Intradermal Method**: For those with a negative subcutaneous node tuberculin test, inject 0.1 ml of BCG (containing 0.05–0.075 mg of bacteria) intradermally at the outer edge of the left deltoid muscle (avoid subcutaneous injection). Newborns under 2 months without a history of subcutaneous node exposure may skip the tuberculin test. For 6 weeks after vaccination, children should avoid contact with subcutaneous node patients to prevent pestilence infection before immunity develops. Three to four weeks after BCG vaccination, a firm red papule may appear at the injection site, gradually forming a small pustule or ulcer, which dries and scabs over, healing within 1–2 months. In more severe reactions, the center of the papule may necrotize, and local lymph nodes may develop cold abscesses, which can rupture into deeper ulcers and heal slowly.
(2) **Scarification Method**: Place a drop of BCG (50–75 mg/ml) on the outer edge of the left deltoid muscle and make a 1–1.5 cm "#"-shaped scratch on the skin, ideally causing redness without bleeding. Gently spread the vaccine over the scratch. After the vaccine dries (about 10 minutes), the sleeve can be worn. This method is simple, easy to promote, causes mild local reactions, and rarely triggers lymph node reactions.
(3) **Oral Method**: Limited to infants under 2 months old. When BCG was first developed by Calmette, it was administered orally to newborns because their intestinal mucosal tissue was not fully developed, allowing BCG to pass through and enter the mesenteric lymphatic system to induce immunity. The oral BCG method is now rarely used.
Among the three methods, the intradermal and oral methods yield higher positive conversion rates. The intradermal method provides longer-lasting immunity than the scarification method. Except for infants under 2 months, a tuberculin skin test (usually 5 subcutaneous node tuberculin units) should be performed before vaccination.
**Contraindications for BCG Vaccination**: Positive subcutaneous node tuberculin reaction, fever, diarrhea, eczema at the injection site, systemic skin diseases, recent acute pestilence (within 1 month), allergic diseases, severe liver, kidney, or heart disease, premature infants, low-birth-weight newborns, and birth-injured infants should not be vaccinated. Children with immunodeficiency may develop fatal disseminated BCG disease and must not be vaccinated.
5. Chemoprophylaxis refers to the administration of isoniazid to prevent subcutaneous node disease, which may be considered under the following circumstances: ① infants and young children exposed to parents with open pulmonary subcutaneous node disease; ② children with recent conversion of tuberculin test from negative to positive (indicating natural infection); ③ infants, young children, and preschool-aged children showing a strongly positive tuberculin reaction; ④ children with a positive tuberculin test and early symptoms of subcutaneous node poisoning but normal lung X-ray findings; ⑤ children with a positive tuberculin reaction who require corticosteroid treatment for other diseases; ⑥ children with a positive tuberculin reaction after contracting measles or whooping cough. The prophylactic dose is 10mg/(kg·d), with a treatment course of 6 months to 1 year. Prophylaxis can achieve three effects: ① preventing active subcutaneous node disease in children; ② preventing the reactivation of subcutaneous node disease during adolescence; ③ preventing the occurrence of extrapulmonary subcutaneous node disease.




