| disease | Mitral Regurgitation |
| alias | Mitral Insufficiency |
The mitral valve consists of four components: the valve leaflets, the annulus, the chordae tendineae, and the papillary muscles. Structural abnormalities or dysfunction in any of these can lead to mitral insufficiency.
bubble_chart Pathogenesis
The main pathophysiological change in mitral regurgitation is that the mitral valve backflow increases the load on the left atrium and the diastolic load on the left ventricle. When the left ventricle contracts, blood flows from the left ventricle into the aorta and the left atrium, which has lower resistance. The backflow volume into the left atrium can exceed 50% of the left ventricular stroke volume. In addition to receiving blood from the pulmonary veins, the left atrium also receives backflow blood from the left ventricle. Therefore, the increase in left atrial pressure can lead to elevated pulmonary venous and pulmonary capillary pressures, resulting in dilation and congestion. Meanwhile, the left ventricle experiences increased diastolic volume load, leading to left ventricular enlargement. In chronic cases, early compensatory mechanisms increase stroke volume and ejection fraction, and left ventricular end-diastolic volume and pressure may not rise, allowing the condition to remain asymptomatic. However, during decompensation, stroke volume and ejection fraction decrease, while left ventricular end-diastolic volume and pressure significantly increase, clinically manifesting as pulmonary congestion and systemic hypoperfusion, signs of left heart failure. In advanced stages, pulmonary hypertension and total heart failure may occur.
In acute mitral regurgitation, a sudden influx of backflow blood into the left atrium can cause a sharp rise in left atrial and pulmonary venous pressures, leading to acute pulmonary edema.
bubble_chart Pathological ChangesAmong patients with chronic onset, those caused by valve leaflet damage due to wind-dampness heat are the most common, accounting for one-third of all patients with mitral regurgitation, and are more prevalent in males. The pathological changes primarily involve inflammation and fibrosis, leading to hardening, shortening, deformation, adhesion, and fusion of the valve leaflets, as well as fusion and shortening of the chordae tendineae. Approximately 50% of patients also have mitral stenosis. Mitral regurgitation may also occur due to: ① Coronary {|###|} atherosclerotic heart disease (coronary heart disease): Myocardial infarction and chronic myocardial ischemia affecting the papillary muscles and adjacent ventricular wall myocardium, resulting in papillary muscle fibrosis with dysfunction. ② Congenital malformations: Mitral valve cleft, most commonly seen in endocardial {|###|} cushion defects or corrected transposition of the great arteries; endocardial {|###|} fibroelastosis; parachute mitral valve deformity. ③ Mitral annular calcification: An idiopathic degenerative {|###|} condition, more common in elderly female patients. Additionally, patients with hypertension, Marfan syndrome, chronic renal failure, and secondary hyperparathyroidism are also prone to mitral annular calcification. ④ Left ventricular enlargement: Significant left ventricular enlargement due to any {|###|} disease cause can lead to mitral annular dilation and lateral displacement of the papillary muscles, impairing leaflet coaptation and resulting in mitral regurgitation. ⑤ Mitral valve prolapse syndrome (see below). ⑥ Other rare {|###|} disease causes: Connective tissue disorders such as systemic lupus erythematosus, rheumatoid arthritis, etc.; hypertrophic obstructive cardiomyopathy; ankylosing spondylitis.
Acute mitral regurgitation is often caused by chordae tendineae rupture, valve {|###|} damage or rupture, papillary muscle necrosis or rupture, or dehiscence after prosthetic valve replacement. It may occur in infective endocarditis, acute myocardial infarction, penetrating or blunt chest trauma, and spontaneous chordae tendineae rupture.bubble_chart Clinical Manifestations
(1) Symptoms Normally, it can take up to 20 years from the initial wind-dampness-related carditis to the appearance of obvious symptoms of mitral regurgitation; once heart failure occurs, the progression is rapid. Patients with grade I mitral regurgitation may have no obvious symptoms or only grade I discomfort. Common symptoms of severe mitral regurgitation include exertional dyspnea, fatigue, orthopnea, and a significant decrease in exercise tolerance. Hemoptysis and embolism are rare. In the advanced stage of right heart failure, congestive hepatomegaly with tenderness, ankle edema, pleural effusion, or ascites may occur. In acute cases, acute left heart failure or pulmonary edema can develop rapidly.
(2) Signs
1. Cardiac auscultation A blowing systolic murmur can be heard at the apex, with an intensity of 3/6 or higher, often radiating to the left axilla and weakening during inspiration. The murmur is high-pitched when the regurgitation volume is small, and rough when the valve membrane is thickened. If the anterior leaflet is predominantly damaged, the murmur radiates to the left axilla or left scapula; if the posterior leaflet is predominantly damaged, the murmur radiates to the base of the heart. A systolic thrill may accompany the murmur. The first heart sound at the apex is diminished or obscured by the murmur. Due to the shortened left ventricular ejection period, the aortic valve closes prematurely, resulting in splitting of the second heart sound. Patients with severe mitral regurgitation may exhibit a low-pitched third heart sound. The presence of an opening snap suggests coexisting mitral stenosis but does not rule out mitral regurgitation. In severe mitral regurgitation, the large volume of blood flow during diastole leads to relative mitral stenosis, resulting in a low-pitched, short diastolic intermediate-stage [second-stage] murmur at the apex. With pulmonary hypertension, the second heart sound at the pulmonary valve area is accentuated.
2. Other signs Blood pressure is normal, but the pulse is relatively thin. The cardiac border expands to the left and downward, and a localized systolic heaving impulse can be palpated at the apex, indicating left ventricular hypertrophy and dilation. With pulmonary hypertension and right heart failure, there may be jugular vein distension, hepatomegaly, and lower limb edema.bubble_chart Auxiliary Examination
(1) X-ray examination: In grade I mitral regurgitation, no obvious abnormalities may be found. In severe cases, the left atrium and left ventricle are significantly enlarged, and a markedly enlarged left atrium may displace and compress the esophagus. When pulmonary hypertension or right heart failure occurs, the right ventricle enlarges. Pulmonary venous congestion, pulmonary interstitial edema, and Kerley B lines may be observed. Calcification of the mitral valve leaflets and annulus is often present. Left ventriculography can quantify mitral regurgitation.
(2) Electrocardiogram (ECG) examination: In grade I mitral regurgitation, the ECG may be normal. In severe cases, left ventricular hypertrophy and strain may be present; with pulmonary hypertension, manifestations of left and right ventricular hypertrophy may appear. Chronic mitral regurgitation with left atrial enlargement is often accompanied by atrial fibrillation. In sinus rhythm, the P wave widens and appears bifid, indicating left atrial enlargement.
(3) Echocardiography: This is the most accurate non-invasive diagnostic method for detecting and quantifying mitral regurgitation. Two-dimensional echocardiography shows enhanced reflections and thickening of the anterior and posterior mitral valve leaflets, with poor coaptation during systole. In cases of chordae tendineae rupture, the mitral valve may exhibit a flail-like change, with the leaflet appearing as a "gooseneck" hooking into the left atrium during systole and a "whiplash" motion toward the left ventricle during diastole on the long-axis view. M-mode echocardiography reveals an increased EF slope of the anterior mitral leaflet during diastole and increased leaflet motion amplitude; left atrial enlargement with excessive expansion during systole; and excessive motion of the interventricular septum. Doppler ultrasound shows systolic regurgitation into the left atrium. Left heart contrast echocardiography demonstrates contrast medium flowing back from the left ventricle to the left atrium during systole (Figure 1).
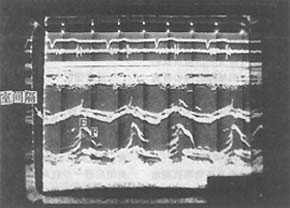
Figure 1: M-mode echocardiogram of mitral regurgitation
showing an increased EF slope and increased interventricular septal motion
(4) Radionuclide examination: Radionuclide blood pool imaging shows enlargement of the left atrium and left ventricle, with increased left ventricular end-diastolic volume. In pulmonary hypertension, the pulmonary trunk and right ventricle may appear enlarged.
(5) Right heart catheterization: Right ventricular, pulmonary artery, and pulmonary capillary wedge pressures are elevated, with increased pulmonary vascular resistance. Left heart catheterization reveals elevated left atrial pressure, a prominent v wave on the pressure curve, and reduced cardiac output.
The clinical diagnosis is primarily based on the typical blowing systolic murmur in the apical area, along with left atrial and left ventricular enlargement, and echocardiography can confirm the diagnosis.
bubble_chart Treatment Measures
(1) Medical Treatment Appropriately avoid excessive physical labor and strenuous exercise, restrict sodium intake, and protect cardiac function; for rheumatic heart disease, actively prevent streptococcal infections and wind-dampness activity, as well as infective endocarditis; use diuretics appropriately; vasodilators, especially afterload-reducing vasodilators, can reduce regurgitation volume and increase cardiac output by lowering left ventricular ejection resistance, thereby producing beneficial hemodynamic effects. Chronic patients may use angiotensin-converting enzyme inhibitors. Acute cases may be treated with intravenous sodium nitroprusside, nitroglycerin, or phentolamine. Digitalis drugs are suitable for patients with heart failure and are more effective for those with atrial fibrillation. Advanced-stage heart failure patients may use anticoagulants to prevent thromboembolism.
(2) Surgical Treatment Long-term follow-up studies indicate that surgical treatment for mitral regurgitation significantly improves cardiac function compared to drug therapy; even in patients with combined heart failure or atrial fibrillation, surgical outcomes are markedly superior to medical treatment. Valve repair surgery has lower mortality rates, higher long-term survival rates, and lower thromboembolism incidence compared to artificial valve replacement.
1. Preoperative Preparation Before surgery, left and right cardiac catheterization and left ventriculography should be performed. These tests greatly aid in diagnosing mitral regurgitation and distinguishing primary myocardial disease from functional mitral regurgitation; hemodynamic assessments help evaluate the severity of the affected valve leaflets; coronary angiography can determine whether the patient requires concurrent coronary artery bypass grafting, as combined coronary artery disease increases surgical mortality and complications.
2. Surgical Indications ① Acute mitral regurgitation; ② Heart function grades 3–4, after aggressive medical treatment; ③ No obvious clinical symptoms or heart function at grade 2 or below, but auxiliary tests indicate progressive cardiac enlargement and a decline in left ventricular ejection fraction. Echocardiography showing a left ventricular end-systolic diameter of 50mm or end-diastolic diameter of 70mm, with an ejection fraction ≤50%, warrants early surgical intervention.
3. Types of Surgery ① Valve Repair Surgery: Maximally preserves the natural valve. Suitable for mitral prolapse due to valve laxity; excessively long or ruptured chordae; localized rheumatic mitral lesions with pliable, non-shrunken anterior leaflets and chordae that are fibrotic or calcified but not contracted; localized infective endocarditis vegetations or perforations with minimal or mild anterior leaflet damage. ② Artificial Valve Replacement: Replacement valves include mechanical and biological valves. Mechanical valves include ball valves, floating disc valves, and tilting disc valves, with the advantage of high durability but a high thromboembolism risk, requiring lifelong anticoagulation. Within 10 years post-surgery, mortality and morbidity due to thromboembolism from insufficient anticoagulation or bleeding from excessive anticoagulation can reach 50%. Additionally, mechanical valves have eccentric blood flow, higher flow resistance, and greater transvalvular pressure gradients. Biological valves include porcine aortic valves, bovine pericardial valves, and homograft dura mater valves, with the advantages of lower thromboembolism rates, no need for lifelong anticoagulation, and central blood flow similar to natural valves, but they are less durable than mechanical valves. Degenerative calcification and damage may occur after 3–5 years, and about 50% require re-replacement after 10 years.
Young patients and those at high risk for atrial fibrillation or thromboembolism requiring anticoagulation should opt for mechanical valves; if the valve annulus is small, a valve with better hemodynamic performance is preferred. For patients with bleeding tendencies or contraindications to anticoagulation, as well as young women planning pregnancy post-surgery, biological valves are recommended.
The complications in chronic patients are similar to those of mitral stenosis but appear later. Infective endocarditis is more common, while embolism is rare. In both acute and chronic patients, when chordae tendineae rupture occurs, acute left heart failure or even acute pulmonary edema may develop rapidly, leading to a poorer prognosis.
The murmur of mitral regurgitation should be differentiated from the following conditions of apical systolic murmurs:
(1) Relative mitral regurgitation: This can occur in hypertensive heart disease, aortic regurgitation of various causes, myocarditis, dilated cardiomyopathy, anemic heart disease, etc. Due to significant enlargement of the left ventricle or mitral annulus, relative mitral regurgitation occurs, leading to an apical systolic murmur.
(2) Functional apical systolic murmur: Approximately half of normal children and adolescents may have a precordial systolic murmur, with an intensity of 1–2/6, short duration, soft quality, not obscuring the first heart sound, and no atrial or ventricular enlargement. It may also be seen in high-output states such as fever, anemia, and hyperthyroidism, and the murmur disappears once the underlying cause is resolved.
(3) Ventricular septal defect: A harsh holosystolic murmur can be heard at the left sternal border in the 3rd–4th intercostal spaces, often accompanied by a systolic thrill. The murmur radiates to the apex, and the apical impulse is heaving. Electrocardiography and X-ray show left and right ventricular enlargement. Echocardiography reveals an interruption in the ventricular septum, and contrast echocardiography can confirm left-to-right shunting at the ventricular level.
(4) Tricuspid regurgitation: A localized blowing holosystolic murmur is heard at the lower left sternal border. The murmur intensifies during inspiration due to increased venous return and weakens during expiration. In pulmonary hypertension, the pulmonary component of the second heart sound is accentuated, and the jugular venous v-wave is prominent. There may be hepatic pulsation and enlargement. Electrocardiography and X-ray show right ventricular hypertrophy. Echocardiography can confirm the diagnosis.
(5) Aortic stenosis: A loud, harsh systolic murmur can be heard at the aortic valve area or apex, radiating to the neck and accompanied by a systolic thrill. An early systolic ejection click may be present, and the apical impulse is heaving. Electrocardiography and X-ray show left ventricular hypertrophy and enlargement. Echocardiography can confirm the diagnosis.




