| disease | Cerebral Palsy |
| alias | Spastic Paralysis, Litter's Disease |
Cerebral palsy (also known as spastic paralysis or Little's disease) refers to a group of conditions characterized by spastic paralysis caused by brain disorders. Patients often experience intellectual developmental disorders, ataxia, speech difficulties, and other impairments. Surgical intervention is only effective for a minority of patients. Rehabilitation therapy, including physical therapy, functional training, as well as speech and occupational therapy, serves as the primary treatment approach. The incidence rate of cerebral palsy in China remains unknown, while in the United States it is 7 per 100,000, and in the UK, it ranges from 1.2% to 2.5%.
bubble_chart Etiology
Any condition that can cause damage to brain tissue can be a contributing factor to the disease.
(1) Prenatal factors: Congenital brain dysplasia is often caused by the mother contracting rubella and other viral infections during pregnancy, especially in the first three months of pregnancy. It is often accompanied by other congenital abnormalities such as internal visual obstruction, deafness, congenital heart disease, etc. Fetal erythroblastosis, which causes severe neonatal jaundice, can lead to injury to the basal ganglia, resulting in kernicterus.
Pregnancy toxemia can cause intracranial or subdural hemorrhage in the fetus. Severe drops in maternal blood pressure can lead to fetal cerebral thrombosis; maternal syncope episodes, barbiturate overdose, hemorrhagic shock, trauma, or burns—all of which cause drops in blood pressure—can damage the fetal brain tissue. Malnutrition can also affect fetal brain development. Generally speaking, congenital factors leading to cerebral palsy are mostly bilateral and symmetrical.
(2) Perinatal factors: These include brain injury and cerebral hypoxia caused by birth trauma, accounting for 37% of the causes of cerebral palsy. The use of forceps to forcibly pull the fetal head can rupture neck veins, leading to intracranial hemorrhage. Cerebral hypoxia can result from the inappropriate use of sedatives or anesthetics during the second stage of labor. A cord around the neck can cause passive cerebral congestion. Prolonged asphyxia and cyanosis after fetal delivery are also prone to cause cerebral palsy. Placental rupture and placenta previa can also lead to fetal brain injury. Premature labor is likely to cause cerebral hemorrhage because the blood vessels in the brains of premature infants are underdeveloped and fragile. During childbirth, the sudden transition from the high-pressure uterus to the external environment results in significant pressure changes, leading to vascular rupture. Premature infants are also particularly sensitive to asphyxia.(3) Postnatal factors: Cerebral palsy caused by infectious encephalitis is often progressive. However, secondary encephalitis or post-infectious encephalitis (such as post-measles encephalitis) manifests as non-progressive and can largely recover with treatment. High fever and convulsions, especially in the first few weeks after birth, often cause intracranial hemorrhage. According to disease cause statistics, birth injury accounts for 13%, hypoxia for 24%, premature labor for 32%, congenital defects for 11%, and postnatal factors for 7%. Among the patients, 30% of their mothers had a history of 1 to 5 late abortions.
bubble_chart Pathological Changes
The pathological changes of cerebral palsy occur in the brain. However, many patients show no structural abnormalities in brain tissue, and the changes often do not correspond to the severity of clinical manifestations. Pathological alterations include: ① underdevelopment of certain brain regions; ② scarring and hardening of brain gyri; ③ localized softening or cyst-like changes in periventricular areas; ④ porencephaly; ⑤ subarachnoid or subdural hematomas.
bubble_chart Clinical ManifestationsClassification. Minear (1958) categorized cerebral palsy into seven types based on the nature and characteristics of paralysis in patients, which greatly aids in understanding the lesion sites, symptoms, and formulating treatment plans. The details are as follows:
(1) Spastic Type. The most common, accounting for about 60%. Pathological changes occur in the motor cortex of the brain, where part of the tissue is replaced by glial tissue, accompanied by pyramidal tract degeneration. Hemiplegia is frequently observed. It may also affect the trunk and facial muscles. Examination reveals: ① Muscle weakness. Due to uneven distribution of weakened muscles in the limbs, muscle imbalance occurs during contraction. ② Spastic muscles are stiff, and passive joint movement feels resistant, accompanied by hyperactive tendon reflexes. ③ Dysfunction and loss of control in voluntary movements. ④ Deformities. When spasticity and muscle imbalance are severe, fixed deformities may develop, such as flexion contracture of the elbow, pronation of the forearm, flexion of the wrist, flexion and adduction of the thumb into the palm; adduction of the hip, flexion of the knee, and equinus deformity of the ankle, leading to a typical "scissors" gait when walking. ⑤ Abnormal intellectual development.
(2) Athetoid Type. Characterized by involuntary, worm-like muscle movements in the limbs or trunk, accounting for about 15%. Lesions are located in the striatum and may involve the caudate nucleus, lentiform nucleus, and globus pallidus. These involuntary movements can be slow or fast, often accompanied by varying degrees of increased muscle tone, severely affecting speech, chewing, and swallowing. Involuntary contractions of facial muscles may produce characteristic "grimacing." Some patients have normal intelligence but cannot express themselves due to loss of control over vocal and facial muscles.
(3) Ataxic Type. Pathological changes occur in the cerebellum, accounting for about 5%. Patients exhibit muscle weakness, hypotonia, ataxia, and easy fatigue, sometimes with tremor that disappears at rest. Patients often display dysmetria, asynergia, and dyssynergia, and balance sensation may also be impaired.(4) Rigid Type. Accounts for about 5%. Caused by widespread lesions in the motor areas of the central nervous system, often due to hypoxia or minor, diffuse bleeding in brain tissue during childbirth. If rigidity is continuous, it is called "lead-pipe" type; if intermittent, "cogwheel" type. Opisthotonos may sometimes occur.
(5) Tremor Type. Commonly manifests as flexion-extension movements of the fingers or toes but may affect entire limbs or even the trunk. Tremor rhythm can be fast or slow, and involuntary movements can be coarse or fine. Tremor frequency may increase during voluntary activities.
(6) Hypotonic Type. Rare. Patients exhibit severely reduced muscle tone and strength, with extremely low speech volume, often only showing lip movements.
(7) Mixed Type. Previously thought to be common, but in fact, it accounts for only about 5%. Patients typically display features of spastic, athetoid, and ataxic types, with one type usually being more prominent.
As the child develops, the type of paralysis may change. At ages 2–4, the spastic type predominates, but as the child grows and matures, extrapyramidal tract dysfunction becomes more apparent, showing athetoid features. This should be considered when formulating treatment plans.
Early diagnosis of sick infants is relatively difficult but crucial to ensure timely treatment. Efforts should be made to establish a diagnosis between 6 months and 1 year of age. First, a detailed inquiry should be conducted regarding the mother's {|###|}pregnancy{|###|} history and {|###|}childbirth{|###|} history. Observe whether the newborn exhibits symptoms such as bulging fontanelle, muscle {|###|}spasm{|###|}, or severe {|###|}jaundice{|###|}. Even if the infant is discharged on schedule without any apparent abnormalities, the presence of these conditions does not definitively rule out problems. Regular follow-ups must be conducted for at least 18 months or longer. If the infant shows signs such as stiff limbs, restricted movement, difficulty spreading legs during diaper changes, reduced or lost muscle tone, excessive or absent limb {|###|}stirred pulse{|###|}, or delayed development, suspicion of this condition should arise.
Illingrowth proposed the following diagnostic criteria: ① Delayed motor development. Delayed motor development in infants with cerebral {|###|}paralysis{|###|} is a significant indicator. ② Hyperactive knee reflexes, ankle clonus, adductor muscle spasms, etc. These signs can often be detected within weeks after birth. ③ When held in the air with the abdomen facing downward, a normal infant will flex the elbows, extend the hips, and bend the knees, whereas an infant with cerebral palsy will exhibit drooping limbs. This can be observed as early as 6 weeks after birth. ④ Neck stiffness, persistent grasping reflex, or inability to open the hands, which may appear after 3 months of age. ⑤ Slow hand opening when given a cubic object, which may manifest after 6 months of age.
bubble_chart Treatment Measures
The treatment of cerebral palsy is multifaceted. The main treatments involve muscle training, speech therapy, and psychological therapy. Orthopedic surgery can only serve as an auxiliary treatment method, and various rehabilitation therapies are required both before and after the surgery.
(1) Non-surgical treatment: ① Muscle training: The principle of muscle training is to educate the child to relax spastic muscles, promote the use of certain muscles, and improve coordination. Repeated and rhythmic movement training is crucial, step by step, to train the child to dress, use the toilet, and walk. ② Use of orthopedic splints. To overcome deformities caused by muscle spasms, splints or gypsum are commonly used tools. The first step is to gradually stretch the shortened muscles and correct the deformity as much as possible. If necessary, correction can be performed under anesthesia, with gypsum used to maintain the limb in an overcorrected position for about 3 months. Afterward, movable braces or splints can be used long-term to prevent recurrence of the deformity. ③ Speech therapy. ④ Occupational training. When the child reaches the age of one year and muscle spasms have partially relaxed after physical therapy, occupational training begins. This includes writing, typing, and some simple manual labor, enabling the patient to become a self-sufficient worker. ⑤ Drug treatment. Drugs have no effect on cerebral palsy, but meprobamate may help control tremors. Sedatives such as chlorpromazine can effectively suppress excessive activity in patients and also aid in physical therapy. Sometimes, antiepileptic drugs can alleviate symptoms like spasms, but close attention must be paid to whether the medication worsens muscle imbalance. Blocking the neuromuscular junction with 1% procaine can inhibit the γ transmission of nerves without affecting the α transmission, thereby reducing muscle spasms. Occasionally, injecting 3% phenol into the nerves to cause permanent damage can relieve spasms in one-third of patients, making training easier.
(2) Surgical treatment: Surgical treatment is only one part of the comprehensive treatment for cerebral palsy. Patients must be strictly selected, and plans must be meticulously formulated. Physical therapy is required both before and after surgery. Generally, children under 5 years old are not suitable for surgical treatment because they are not yet cooperative, examination is difficult, and the extent and consequences of paralysis may not yet be fully apparent.
The surgical methods include the following:
(1) Neurological surgery: ① For patients with athetosis, anterior rhizotomy of the spinal nerves may be considered. Severing the anterior roots of cervical 3 to thoracic 1 spinal nerves can completely eliminate all movements of the upper limbs without affecting sensory function, which may benefit some patients. ② For spastic hemiplegia patients with severe epilepsy, hemispherectomy can reduce the frequency and severity of seizures, making it easier for the patient to undergo training. ③ For severe athetosis-type cerebral palsy, pallidotomy may have some therapeutic effect. The above three surgeries are all destructive and must be strictly indicated; they should not be performed lightly. ④ Peripheral nerve surgery. Commonly, part or all of the nerve支配 an overly spastic muscle is severed to relax the muscle. This surgery is more often performed on the lower limbs.
(2) Muscle and tendon surgery: Includes ① Tendon切断术 or tendon lengthening: Performing tendon切断术 or lengthening on spastic muscles can减轻 their mechanical强力收缩 and improve muscle balance. ② Tendon transfer: In certain areas, relocating the insertion point of muscles that加重畸形 to a new insertion point can alter their function, turning the force that加重畸形 into a动力 that corrects畸形.
(3) Bone and Joint Surgery Including ① Bone lengthening or shortening to correct limb length discrepancy. ② Osteotomy, including wedge osteotomy and rotational osteotomy to correct deformities. ③ Arthrodesis, fixing the joint in a functional position to enhance stability and improve function.
Below is a brief introduction to some common deformities and relatively mature treatment methods.
(1) Treatment of Hand and Wrist Deformities Due to the complex functionality of the hand and wrist joints, only about 4% of patients can achieve functional improvement through surgery. ① If the thumb is flexed and adducted into the palm with spasms, but the patient can still make a fist and open the hand, a thumb metacarpophalangeal joint fusion and abductor pollicis longus shortening procedure can be performed. ② If the wrist and fingers cannot dorsiflex, the thumb is spasmed into the palm, and the flexor digitorum superficialis has grade II spasticity, a thumb metacarpophalangeal joint fusion can be done, transferring the flexor digitorum superficialis through the interosseous membrane to the extensor digitorum and extensor pollicis longus, and moving the flexor carpi ulnaris to the extensor carpi radialis brevis. ③ For severe flexion spasms of the fingers, the flexor carpi ulnaris can be transferred to the extensor digitorum, and the flexor carpi radialis to the extensor pollicis longus, along with thumb metacarpophalangeal joint fusion. If necessary, the wrist joint can also be fixed in a functional position. ④ For poor wrist flexion function, the brachioradialis can be transferred to the flexor carpi muscles. ⑤ If the hand cannot open due to severe flexion contractures, tendon lengthening and fusion of the thumb metacarpophalangeal joint and wrist joint can be performed. However, this surgery is mainly for cosmetic purposes and has limited functional benefits.
(2) Treatment of Shoulder and Elbow Deformities If shoulder abduction is less than 45° or external rotation is less than 15°, a subscapularis tenotomy and pectoralis major release can be performed. For elbow flexion deformities exceeding 40°, the elbow extensors can be lengthened.
(3) Treatment of Hip Deformities Hip deformities are very common. ① Correction of Hip Flexion-Internal Rotation Deformity: For grade I or II deformities that only occur during walking, the semitendinosus can be transferred to the anterior aspect of the lateral femoral condyle, or an anterior hip soft tissue release can be performed followed by abduction cast immobilization. If the tensor fasciae latae is the main spastic factor, its origin can be relocated posteriorly to the iliac crest to promote external rotation. If the flexion deformity is fixed and cannot be passively corrected, a subtrochanteric or supracondylar rotational osteotomy is required (Figure 1). ② Correction of Hip Flexion Deformity: If the deformity exceeds 45° in children aged 8–11 years, an iliopsoas tenotomy can be performed. If caused by rectus femoris spasticity, its iliac origin can be released. ③ Correction of Hip Adduction Deformity: An obturator neurectomy and adductor tenotomy are often performed (Figure 2). However, the strength of the hip abductors must be evaluated preoperatively. A diagnostic obturator nerve block can be done first to assess abductor strength. If the gracilis contributes to adduction spasticity, the patient can be placed prone with the hip maximally abducted and the knee flexed. Gradual knee extension will cause hip adduction if gracilis contracture is present. Transecting the muscle at the musculotendinous junction can correct the deformity. ④ Management of Hip Dislocation: Some believe hip dislocation is inevitable in children with spastic paralysis of the lower limbs. For subluxation, an anterior obturator neurectomy, release of contracted adductors or gracilis, and hip abduction casting for 6 weeks followed by abductor strengthening are recommended. If over half of the femoral head is outside the acetabulum in children over 9 years old, a subtrochanteric varus osteotomy is indicated. For chronic dislocations with coxa valga, increased femoral anteversion, and shallow acetabulum: - Non-ambulatory children may not require treatment. - Ambulatory children may undergo: ① Hip arthrodesis; ② Femoral osteotomy to correct angulation and rotation, plus pelvic osteotomy to deepen the acetabulum; ③ Subtrochanteric abduction osteotomy to use the pelvis as a weight-bearing support.
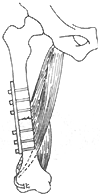
Figure 1 External Rotational Osteotomy of the Femur
Transfer of the semitendinosus to the lateral aspect of the femur (indicated by dashed lines) to correct internal rotation deformity.
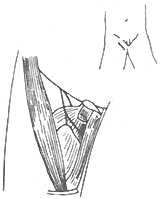
Figure 2 Adductor Tenotomy and Obturator Neurectomy
The figure shows the transected adductor longus muscle, with the anterior branch of the obturator nerve in the front and the posterior branch at the back.
(IV) Treatment of Knee Joint Deformities When determining the treatment plan, attention should be paid not only to the local issues of the knee but also to deformities of the hip and ankle joints, particularly the muscles that can affect the movement of both joints, such as the rectus femoris, gracilis, biceps femoris, semitendinosus, semimembranosus, and gastrocnemius. If the knee flexion deformity cannot be passively corrected, surgery is required. The degree of active knee extension and the position of the patella should first be examined. It is often found that the patella is displaced upward and the quadriceps tendon is elongated. An upwardly displaced patella weakens the knee-extending force of the quadriceps and can lead to knee contracture. Posterior knee joint capsulotomy and "Z"-lengthening of the hamstrings can be performed. Some advocate for distal displacement of the patellar tendon insertion (Figure 3). Others recommend releasing the patellar retinaculum and relocating the hamstring insertions to the distal femur (Figure 4) to correct knee flexion deformity. However, the strength of the gastrocnemius must be considered, as after such a transfer, the gastrocnemius becomes the sole knee flexor. If an Achilles tendon lengthening has been performed or the gastrocnemius is too weak, the knee may lose flexion ability. Therefore, some have modified this procedure to include releasing the gracilis, transferring the semitendinosus to the medial femoral condyle, lengthening the semimembranosus, and leaving the biceps femoris in place. Knee flexion contracture and upward patellar displacement can lead to chondromalacia patellae, causing knee pain, so some also advocate for patellectomy.
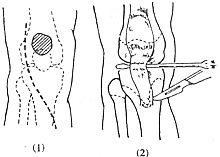
(1) Incision (2) Freeing the patellar ligament
Figure 3 Distal Displacement of the Patellar Tendon Insertion
(1) Knee flexion caused by hamstring contracture
(2) Transferring the medial and lateral hamstrings to the medial and lateral femoral condyles, converting their biarticular function to monoarticular function
Figure 4 Conversion of Hamstring Biarticular Function to Monoarticular Function
(V) Treatment of Foot Deformities
1. Correction of Equinus Deformity ① Tibial Nerve Branch Neurectomy: Cutting the motor branches to the gastrocnemius or soleus, or both, is effective for correcting spastic equinus deformity, can reduce ankle clonus, and aids walking (Figure 6). Preoperative examination must determine whether ankle clonus is caused by the gastrocnemius or soleus. If ankle clonus disappears when the knee is flexed, it indicates the gastrocnemius is the cause; otherwise, it is the soleus. This can guide the choice of which tibial nerve branch to cut. ② Triceps Surae Release. Spastic equinus has two scenarios: ① Equinus present with the knee extended but correctable when the knee is flexed to 90°; ② Equinus persists whether the knee is flexed or extended. The first scenario indicates gastrocnemius contracture, corrected by transferring its origin from the distal femur to the proximal tibia. The second scenario indicates contracture of both the gastrocnemius and soleus, requiring Achilles tendon lengthening. ③ Anterior Displacement of the Achilles Tendon Insertion: Since equinus often recurs after Achilles tendon lengthening, some relocate the Achilles tendon insertion to the dorsal aspect of the calcaneus near the posterior edge of the talocalcaneal joint. This reduces the leverage of the triceps surae and is more effective for non-fixed equinus deformities.

(1) Exposing the gastrocnemius tendon and the gastrocnemius branch of the tibial nerve

(2) Cut the gastrocnemius tendon and one of the tibial nerve branches to the gastrocnemius muscle
Figure 5 Gastrocnemius Tendonotomy with Partial Neurectomy of the Gastrocnemius Branch of the Tibial Nerve
2. Correction of Varus and Valgus Deformities For fixed varus or valgus deformities, correction can be achieved through calcaneal wedge osteotomy or subtalar arthrodesis to enhance foot stability. However, muscle imbalance must also be addressed. Typically, varus deformity results from contractures of the tibialis anterior and posterior muscles, while valgus deformity arises from contractures of the peroneus longus and brevis muscles. Therefore, these muscles can be transferred to antagonistic positions to correct the deformity. Alternatively, some advocate splitting the tibialis anterior or posterior tendon into two parts: one half remains at its original insertion, while the other half is sutured to the peroneus brevis or cuboid bone to correct varus deformity. Before surgery, it is essential to identify which muscle primarily contributes to the deformity and split that specific muscle. For instance, if varus deformity is solely due to tibialis posterior contracture, a "Z"-lengthening of the tibialis posterior tendon or muscle elongation may be performed.
3. Correction of Calcaneus Foot This is rare and often results from excessive Achilles tendon lengthening or concurrent neurectomy of the tibial nerve motor branches. The tibialis anterior, tibialis posterior, and gastrocnemius muscles can be transferred to the Achilles tendon. Some also recommend talectomy.
4. Correction of Claw Toes The motor branch of the lateral plantar nerve innervates all interosseous muscles (except those between the fourth and fifth metatarsals), the second, third, and fourth lumbricals, and the adductor hallucis. The first lumbrical and flexor hallucis brevis are innervated by the medial plantar nerve. Correction can be achieved through neurectomy of the motor branch, capsulotomy of the metatarsophalangeal joints, and tenotomy of the flexor hallucis brevis.
5. Correction of Forefoot Adduction This often results from contracture of the abductor hallucis muscle and can be corrected by tenotomy of this muscle.
(6) Management of Spinal Deformities Approximately 20–25% of patients may develop (paralytic) scoliosis. If the curvature is less than 30° in standing or sitting positions and non-progressive, bracing may be used. Surgical intervention may be considered under the following circumstances: ① Thoracic scoliosis exceeding 60° with cardiopulmonary complications; ② Imbalance due to thoracolumbar scoliosis in sitting positions, affecting upper limb function; ③ Rarely, surgery may be performed for cosmetic reasons.
Since brain tissue damage cannot be repaired or replaced, a complete cure is impossible. However, even just to alleviate symptoms, both patients and rehabilitation trainers require immense patience. Many patients need lifelong specialized care. Yet, a small number can achieve significant improvement, enabling them to live independently or even become self-sufficient. Early functional training (such as reflexive crawling and rolling movements) before the age of 1 generally leads to a relatively better prognosis.
It is generally estimated that 25% of children with cerebral palsy can attend regular elementary schools, while another 25% require lifelong care and cannot engage in any work. Another 25% may barely manage daily life but cannot receive education, and the remaining 25% need functional exercises and vocational training in special schools. Long-term follow-up studies of some children found that 60% could eventually perform paid work. Orthopedic surgery can help restore partial function in affected children.

