| disease | Hemorrhagic Shock |
| alias | Hemorrhagic Shock |
A large amount of blood loss leading to shock is called hemorrhagic shock, commonly seen in bleeding caused by trauma, peptic ulcer bleeding, rupture of esophageal varices, and bleeding caused by gynecological and obstetric diseases. Whether shock occurs after blood loss depends not only on the amount of blood loss but also on the speed of blood loss. Shock often occurs in cases of rapid and massive blood loss (exceeding 30-35% of total blood volume) that is not promptly replenished.
bubble_chart Pathogenesis
Microcirculation disorder (ischemia, congestion, disseminated intravascular coagulation) leads to insufficient blood perfusion in the microcirculation stirred pulse, causing functional and metabolic disturbances in vital organs due to hypoxia, which is a common pattern in all types of shock. The changes in microcirculation during shock can be roughly divided into the late stage [third stage], namely the microcirculation ischemia phase, the microcirculation congestion phase, and the microcirculation coagulation phase.
(1) Microcirculation Ischemia Phase (Ischemic Hypoxia Phase)
The characteristics of microcirculation changes in this phase are: ① The micro stirred pulse, post-micro stirred pulse, and precapillary sphincter contract, leading to a sharp decrease in microcirculation perfusion and a drop in pressure; ② Micro veins and small veins are less sensitive to Black Catechu phenol amines and contract less; ③ Arteriovenous anastomoses may open to varying degrees, allowing blood to flow directly from the micro stirred pulse through arteriovenous anastomoses into small veins.
The key change causing microcirculation ischemia is the strong excitation of the sympathetic nervous system-adrenal medulla system. Different types of shock can cause sympathetic-adrenal medullary shock and cardiogenic shock through different mechanisms. A decrease in cardiac output and stirred pulse blood pressure can excite the sympathetic-adrenal medulla system through the sino-aortic reflex; in most cases of internal toxin shock, internal toxins can directly stimulate the sympathetic-adrenal medulla system, causing it to become strongly excited.
The overall effect of sympathetic nerve excitation and increased release of Black Catechu phenol amines on the heart blood vessel system is to increase peripheral resistance and cardiac output. However, the response of blood vessels in different organs varies greatly. Blood vessels in the skin, abdominal viscera, and kidneys, due to their rich sympathetic vasoconstrictor fiber innervation and the predominance of α receptors, contract in these areas' small stirred pulses, small veins, micro stirred pulses, and precapillary sphincters when sympathetic nerves are excited and Black Catechu phenol amines increase. Among these, the micro stirred pulse has the densest distribution of sympathetic vasoconstrictor fibers, and the precapillary sphincter is most responsive to Black Catechu phenol amines, hence they contract most strongly. As a result, precapillary resistance significantly increases, microcirculation perfusion sharply decreases, and the average blood pressure in capillaries significantly drops, with only a small amount of blood flowing through direct pathways and a few true capillaries into micro veins and small veins, causing severe ischemic hypoxia in tissues. Cerebral blood vessels have the least sympathetic vasoconstrictor fiber distribution and low α receptor density, so their diameter may not change significantly. Although coronary stirred pulses also have sympathetic nerve innervation and both α and β receptors, sympathetic nerve excitation and increased Black Catechu phenol amines can lead to enhanced heart activity, increased metabolic levels, and thus an increase in vasodilating metabolites, especially adenosine, causing coronary stirred pulses to dilate. Sympathetic excitation and reduced blood volume can also activate the renin-angiotensin-aldosterone system, with angiotensin II having a strong vasoconstrictive effect, including on coronary stirred pulses.Additionally, increased Black Catechu phenol amines can stimulate platelets to produce more thromboxane A2 (thromboxane A2, TXA2), and TXA2 also has a strong vasoconstrictive effect.
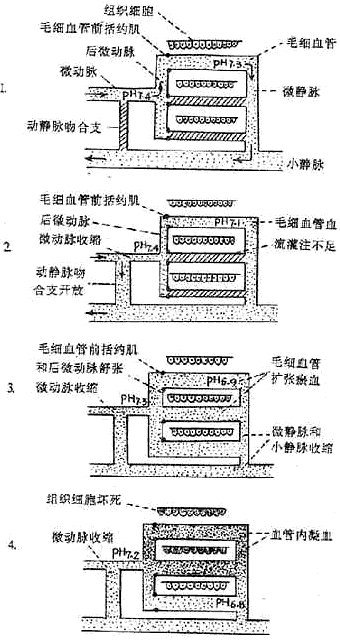
Figure 1 Schematic diagram of the development process of microcirculation disorder
1. Normal situation
(1) Arteriovenous anastomoses are closed.
(2) Only 20% of capillaries are alternately open, with blood perfusion.
(3) The opening and closing of capillaries are regulated by the relaxation and contraction of the precapillary sphincter.
2. Microcirculation Ischemia Phase
(1) Sympathetic nerve excitation and increased secretion of adrenaline and noradrenaline cause contraction of small stirred pulses, micro stirred pulses, post-micro stirred pulses, and precapillary sphincters.
(2) The arteriovenous anastomosis opens, and blood flows directly from the micro stirred pulse into the small vein.
⑶ Insufficient capillary blood perfusion leads to tissue hypoxia.
3. Microcirculation congestion phase
⑴ Small stirred pulse and micro stirred pulse contract, arteriovenous anastomoses remain open, and the amount of blood entering the capillaries is still very small.
⑵ Due to tissue hypoxia, vasodilator substances such as histamine, bradykinin, and hydrogen ions increase, post-micro stirred pulse and precapillary sphincters relax, capillaries open, vascular volume expands, and blood flow within the capillaries becomes very slow.
⑶ Due to sympathetic nerve excitation, the secretion of adrenaline and noradrenaline increases (possibly also the effect of histamine), causing micro veins and small veins to contract, increasing post-capillary resistance, resulting in capillary dilation and congestion.
4. Microcirculation coagulation phase⑴ Due to severe tissue hypoxia and acidosis, capillary walls are damaged and permeability increases, blood within the capillaries becomes concentrated, blood flow stagnates; additionally, blood coagulability increases, resulting in disseminated intravascular coagulation within the microcirculation.
⑵ Due to microthrombus formation, tissue hypoxia and metabolic disorders are further aggravated, intracellular lysosomes rupture, tissue cells necrose, leading to severe dysfunction of various organs.
⑶ Due to coagulation, coagulation factors (such as prothrombin, fibrinogen, etc.) and platelets are largely consumed, fibrin degradation products increase, further reducing blood coagulability; vascular walls are also damaged, leading to widespread bleeding.
And TXA 2 also has a strong vasoconstrictive effect.
Additionally, the lysosomal hydrolase-myocardial depressant factor system also plays a role in the occurrence of microcirculatory ischemia in the first phase of shock. During shock, mainly due to reduced pancreatic blood perfusion causing ischemia, hypoxia, and acidosis, pancreatic exocrine cell lysosomes rupture and release cathepsins, which can then decompose tissue proteins to generate myocardial depressant factor (MDF). After the small peptide MDF enters the bloodstream, besides causing weakened myocardial contraction and inhibiting the phagocytic function of the mononuclear phagocyte system, it can also cause contraction of small blood vessels in the abdominal viscera, further aggravating microcirculatory ischemia in these areas.
The main clinical manifestations of this phase are: pale skin, cold limbs, cold sweating, reduced urine output; because of increased peripheral resistance, systolic pressure may not significantly decrease, while diastolic pressure may increase, pulse pressure decreases, pulse becomes rapid and thin; consciousness is clear, dysphoria and restlessness, etc.
The microcirculatory changes in this phase have certain compensatory significance. Contraction of small stirred pulse in the skin and abdominal organs can both increase peripheral resistance to maintain blood pressure and reduce blood flow to these tissues and organs to ensure blood supply to vital organs such as the heart and brain; increased precapillary resistance, decreased capillary hydrostatic pressure, promoting tissue fluid to enter blood vessels, increasing plasma volume; additionally, opening of arteriovenous anastomoses, venous contraction reduces venous capacity (normally about 70% of blood is in the veins), which can accelerate and increase the return of heart blood, also beneficial for maintaining blood pressure and blood supply to the heart and brain. However, due to insufficient microcirculatory stirred pulse blood perfusion leading to hypoxia in most tissues and organs, it will lead to further development of shock. If detected early, actively rescued, and blood volume replenished in time, reducing excessive stress response, microcirculation and blood pressure can be quickly improved, preventing further deterioration of shock, and turning danger into safety.
The mechanism of microcirculatory changes at this time can be summarized as follows (Figure 2):
(II) Microcirculation congestion phase (congestive hypoxia phase)
During the circulatory ischemic phase of shock, if timely resuscitation and improvement of microcirculation are not performed, the persistent and severe hypoxia in tissues leads to an increase in local vasodilatory substances (such as histamine, kinins, lactic acid, adenosine, etc.), causing the post-micro stirred pulse and precapillary sphincters to dilate, expanding the microcirculation capacity and leading to congestion, which develops into the microcirculatory congestion phase of shock. The characteristics of microcirculatory changes in this phase are: ① The post-micro stirred pulse and precapillary sphincters dilate (due to local acidosis, the responsiveness to Black Catechu phenolamine decreases), a large number of capillaries open, some showing irregular sac-like dilation (formation of micro blood pools), thereby expanding the microcirculation volume; ② Micro veins and small veins have greater tolerance to local acidosis, and Black Catechu phenolamine can still cause them to contract (histamine can also cause the contraction of micro veins and small veins in the liver, lungs, etc.), increasing the post-capillary resistance and slowing down the microcirculatory blood flow; ③ The permeability of microvascular walls increases, plasma exudation occurs, and blood flow stagnates; ④ Due to blood concentration, increased hematocrit, red blood cell aggregation, white blood cell plugging, platelet adhesion, and aggregation, and other hemorheological changes, the microcirculatory blood flow can slow down or even stop. ⑤ Due to microcirculatory congestion and increased pressure, less stirred pulse blood enters the microcirculation (at this time, small stirred pulse and micro stirred pulse remain in a contracted state due to sympathetic nerve action). As a large amount of blood accumulates in the microcirculation, the return of heart blood decreases, further reducing cardiac output and exacerbating the development of shock.
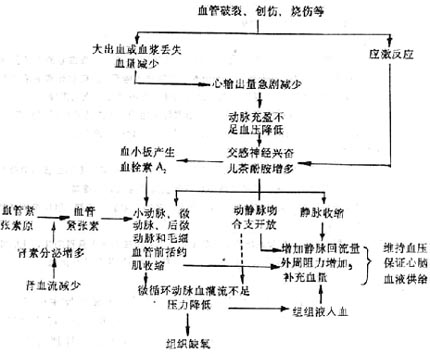
Figure 2 Mechanism of Microcirculation Changes in Ischemic Hypoxia Phase
Due to the aforementioned microcirculation changes, although a large amount of blood accumulates in the microcirculation, the stirred pulse blood perfusion will further decrease. The patient's skin color changes from pale to gradually cyanotic, especially in the lips and fingertips. Because the venous return and cardiac output are further reduced, the patient's veins collapse and fill slowly; the stirred pulse pressure is significantly reduced, the pulse pressure is small, and the pulse is thin and rapid; due to insufficient blood supply to the heart and brain, ATP production decreases, manifesting as weakened cardiac contractility (low heart sounds), apathy, or unconsciousness. Severe cases may lead to heart, kidney, and lung failure. This is a critical state of shock and requires immediate rescue, fluid replacement, relief of small vessel spasms, oxygen administration, and correction of acidosis to unblock the microcirculation and prevent disseminated intravascular coagulation. The mechanism of microcirculation changes at this stage can be summarized as follows (Figure 3):
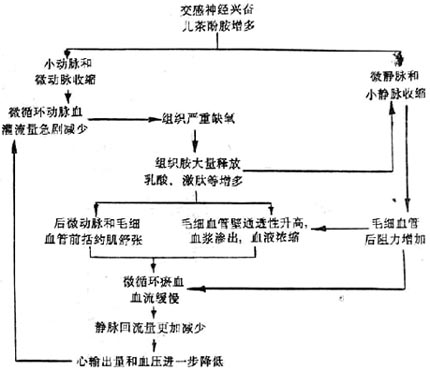
Figure 3 Mechanism of Microcirculation Changes in Congestive Hypoxia Phase
(3) Microcirculation Coagulation Phase (Disseminated Intravascular Coagulation)
The progression from the congestive phase to the coagulation phase of microcirculation is a manifestation of shock deterioration. Its characteristics are: on the basis of microcirculation congestion, fibrin thrombi form within the microcirculation (especially at the venous end of capillaries, venules, and small veins), often accompanied by focal or diffuse bleeding; tissue cells undergo degeneration and necrosis due to severe hypoxia.
Disseminated intravascular coagulation is closely related to shock. The pathological changes caused by disseminated intravascular coagulation and how it induces or exacerbates the development of shock have been discussed in the chapter on "Disseminated Intravascular Coagulation". Here, we briefly summarize how shock leads to disseminated intravascular coagulation.
1. Stress response increases blood coagulability. Shock-inducing factors (such as trauma, burns, hemorrhage, etc.) and shock itself are strong stimuli that can cause a stress response, sympathetic nerve excitation, and increased pituitary-adrenal cortex activity, leading to an increase in platelets and clotting factors in the blood, enhanced platelet adhesion and aggregation, providing the necessary material basis for coagulation.
2. Release and activation of clotting factors. Some shock-inducing factors (such as trauma, burns, etc.) themselves can cause the release and activation of clotting factors. For example, injured tissues can release a large amount of tissue thromboplastin, initiating the extrinsic coagulation process; large-area burns cause massive destruction of red blood cells, and the phospholipids within the red blood cell membrane and ADP released from the destruction of red blood cells promote the coagulation process.
3. Microcirculation disorders, tissue hypoxia, and local increases in histamine, kinins, lactic acid, etc. These substances, on one hand, cause capillary dilation and congestion, increased permeability, slow blood flow, blood concentration, increased red blood cell viscosity, favoring thrombus formation; on the other hand, they damage capillary endothelial cells, expose collagen, activate clotting factor XII, and promote platelet adhesion and aggregation.
4. Hypoxia reduces the function of the mononuclear phagocyte system, preventing the timely clearance of prothrombinase, thrombin, and fibrin. As a result, under the influence of the above factors, disseminated intravascular coagulation occurs.
Once disseminated intravascular coagulation occurs, it will further exacerbate microcirculation disorders and worsen the shock condition, because: ① widespread microvascular blockage further aggravates microcirculation disorders, further reducing the return of heart blood; ② consumption of coagulation substances, activation of secondary fibrinolysis, and other factors cause bleeding, thereby reducing blood volume; ③ soluble fibrin polymers and their degradation products can block the mononuclear phagocyte system, preventing the adequate clearance of internal toxins from the intestines.
Due to the occurrence of disseminated intravascular coagulation and the continuous aggravation of microcirculatory stasis, the severe insufficiency of systemic microcirculatory perfusion caused by decreased blood pressure leads to increasingly severe systemic hypoxia and acidosis; severe acidosis can cause the rupture of lysosomal membranes within cells, releasing lysosomal enzymes (such as proteases) and certain shock factors (such as internal toxins), which can cause severe and even irreversible damage to cells, thereby further exacerbating the functional and metabolic disorders of vital organs including the heart and brain (details to follow). This creates significant difficulties in treatment, hence this stage is also referred to as the refractory stage of shock.
bubble_chart Clinical Manifestations
Insufficient capacity to surpass compensatory functions will result in shock syndrome. A decrease in cardiac output leads to a drop in blood pressure despite peripheral vasoconstriction. Reduced tissue perfusion promotes anaerobic metabolism, leading to increased lactate levels and metabolic acidosis. Redistribution of blood flow maintains blood supply to the brain and heart. Further vasoconstriction can cause cellular damage. Damage to vascular endothelial cells results in fluid and protein loss, exacerbating hypovolemia. Ultimately, multiple organ failure may occur. The intestinal mucosal barrier's defense capability against hemorrhagic shock is compromised, likely serving as a significant mechanism for pneumonia and other infectious complications. Sublethal blood loss exhibits cross-tolerance to the attack of internal toxins, meaning that sublethal blood loss can provide protection against lethal doses of internal toxins.
In many cases, diagnosing bleeding is not too difficult. Both medical history and signs can reflect intravascular volume depletion and adrenergic compensatory responses. However, laboratory tests are not always reliable. This is because, shortly after acute blood loss, fluid shifts may not yet be significant, making it difficult to reflect through blood test indicators. If the blood loss process is slightly prolonged, fluid shifts gradually increase, leading to hemoconcentration, manifested as increased hemoglobin, elevated hematocrit, and an increased ratio of blood urea nitrogen to creatinine. If the blood loss process is prolonged and the volume of blood loss is substantial, especially with a gradual increase in free water loss, serum sodium levels may also rise. In summary, the volume of blood loss in shock should be fully estimated, as clinical underestimation is common and warrants attention.
When blood loss is significant, causing severe hypovolemic shock, and clinically it is difficult to grasp the actual and regular changes, especially when resuscitation fluid therapy fails to show positive effects, consideration should be given to placing a central venous catheter or pulmonary artery catheter for invasive hemodynamic monitoring. Through central pressure monitoring, a decrease in central venous pressure (CVP) and pulmonary artery wedge pressure (PCWP), a reduction in cardiac output, a decrease in venous oxygen saturation (SVO2), and an increase in systemic vascular resistance can be observed.
bubble_chart Treatment Measures
The treatment of hemorrhagic shock involves several critical steps. Firstly, it is essential to ensure airway patency and effective hemostasis. Airway patency is a fundamental requirement for ventilation and oxygenation and must be firmly guaranteed. For patients with severe shock and circulatory failure, moving qi endotracheal intubation should be performed, and mechanical ventilation should be administered. Hemostasis is a crucial measure to prevent the onset and progression of shock. Compression hemostasis is a viable and effective emergency measure; the application of a tourniquet is also highly effective. Two intravenous infusion channels should be established as quickly as possible.
Following the establishment of the infusion channels, immediate and rapid fluid resuscitation should be administered. For severe shock, 1-2 liters of isotonic balanced salt solution should be rapidly infused, followed preferably by cross-matched blood. To save lives, type-specific or O-type red blood cells can be transfused. Especially after the application of balanced salt solution, if the resuscitation requirements are still not met during volume restoration, red blood cells should be transfused to achieve a hemoglobin level above 10g/dl. However, in cases of continuous bleeding, the above method of fluid and blood transfusion is inappropriate because vigorous fluid resuscitation can dislodge clots, increase blood loss, and reduce survival rates. Therefore, the use of hypertonic salt solution for rapid volume expansion, particularly in pre-hospital emergency care, remains controversial.
In the absence of monitoring through central venous catheterization or pulmonary artery catheterization, treatment should be guided by the following clinical indicators: urine output should reach 0.5-1.0 ml/(kg.h), normal heart rate, normal blood pressure, good capillary refill, and normal consciousness.
It is worth noting that after resuscitation for massive blood loss, in addition to blood transfusion to compensate for blood loss, a certain amount of crystalloid and colloid solutions should also be supplemented to meet the needs of fluid separation. Failure to understand this need and merely adopting fluid restriction and diuretic management will exacerbate shock, lead to metabolic acidosis, induce multiple organ dysfunction, and even cause death. Approximately one day later, the body fluid shifts from the separation phase to the diuretic phase, and by increasing diuresis to mobilize excess fluid accumulated outside the blood vessels, the fluid compartments gradually return to the pre-injury normal levels.
1. Actively prevent and treat infections.
2. Properly handle on-site trauma treatment, such as timely hemostasis, analgesia, and warmth preservation.
3. Patients with loss of blood or excessive fluid loss (such as vomiting, diarrhea, hemoptysis, gastrointestinal bleeding, profuse sweating, etc.) should be promptly and appropriately rehydrated or transfused.




