| disease | Tricuspid Valve Downward Shift Malformation |
| alias | Ebstein's Anomaly |
Ebstein's anomaly is a rare congenital heart defect. It was first reported by Ebstein in 1866, hence also known as Ebstein's anomaly. Its incidence accounts for 0.5 to 1% of congenital heart diseases.
bubble_chart Pathogenesis
The hemodynamic changes in Ebstein's anomaly depend on the severity of tricuspid regurgitation, the presence and size of an atrial septal defect, and the degree of right ventricular dysfunction. Due to the dilation of the atrioventricular ring and right ventricle, as well as leaflet deformation, varying degrees of tricuspid regurgitation are common. During right atrial contraction, the right ventricle relaxes, and the atrialized portion of the ventricle also dilates, preventing all the blood from the right atrium from entering the right ventricle. When the right atrium relaxes and the right ventricle contracts, the atrialized right ventricle also contracts. As a result, the right atrium simultaneously receives blood from the systemic veins, the atrialized right ventricle, and regurgitant flow through the tricuspid valve, leading to increased blood volume in the right atrium, atrial dilation, elevated right atrial pressure, and ultimately heart failure. In cases with a patent foramen ovale or atrial septal defect, when right atrial pressure exceeds left atrial pressure, right-to-left shunting occurs, reducing systemic arterial oxygen saturation and resulting in cyanosis and clubbing of the fingers (toes). With an intact atrial septum, during right ventricular contraction, the amount of blood entering the lungs for gas exchange decreases, leading to a reduced arteriovenous oxygen difference. This may cause facial flushing and grade I cyanosis in the fingertips (Figure 3).
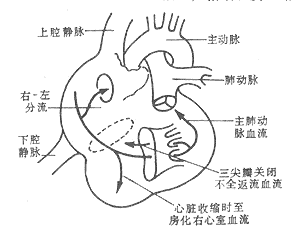
Figure 3 Schematic diagram of the pathophysiology of Ebstein's anomaly.
bubble_chart Pathological Changes
The pathological changes of Ebstein's anomaly vary significantly, with the fundamental lesion being abnormal development of the tricuspid valve leaflets and the right ventricle, accompanied by downward displacement of the septal and posterior leaflets into the right ventricle. These leaflets are attached via chordae tendineae and papillary muscles to the right ventricular wall below the tricuspid annulus. The tricuspid valve leaflets may be enlarged or reduced in size, often thickened, deformed, and shortened. The septal leaflet is most frequently affected, followed by the posterior leaflet, which may be partially absent. Involvement of the anterior leaflet is rare. The anterior leaflet originates from the normal tricuspid annulus and may enlarge like a sail, sometimes with multiple small perforations, attaching to the ventricular wall via shortened and hypoplastic chordae tendineae and papillary muscles. The displaced leaflets divide the right ventricle into two parts: the dilated ventricle above the leaflets, termed the atrialized ventricle, which functions similarly to the right atrium, and the functional right ventricle below the leaflets. The right atrium is enlarged, with fibrotic and thickened walls. The right atrium and the highly dilated, thin-walled atrialized right ventricle form a large chamber that serves to store blood, while the functional right ventricle below the leaflets functions to eject blood. In cases of Ebstein's anomaly, the tricuspid annulus and right ventricle are often severely dilated, and the malformed leaflets typically result in tricuspid regurgitation. If the free edges of the leaflets are partially adherent, the enlarged anterior leaflet may cause obstruction between the atrialized ventricle and the functional right ventricle, leading to varying degrees of tricuspid stenosis. The atrioventricular node and bundle are anatomically normal, but the right bundle branch may be compressed by thickened endocardium, resulting in right bundle branch block. Approximately 5% of cases exhibit an abnormal Kent bundle, manifesting as pre-excitation syndrome. About 50–60% of Ebstein's anomaly cases are associated with a patent foramen ovale or atrial septal defect, leading to right-to-left shunting at the atrial level, reduced arterial oxygen saturation, and clinical cyanosis. Other associated anomalies include pulmonary stenosis, ventricular septal defect, patent ductus arteriosus, tetralogy of Fallot, transposition of the great arteries, aortic coarctation, and congenital mitral stenosis (Figure 4).

Figure 4 Schematic diagram of the pathological anatomy of Ebstein's anomaly
bubble_chart Clinical Manifestations
(1) Symptoms A few patients may present with dyspnea, cyanosis, and congestive heart failure within 1 week after birth. However, most patients gradually develop exertional dyspnea, lack of strength, palpitation, cyanosis, and heart failure after entering childhood. Supraventricular tachycardia can occur in patients of all age groups, and some patients may have pre-excitation syndrome.
(2) Signs Most patients exhibit poor growth and development, with a thin and small physique. About one-third of patients have flushed cheeks resembling mitral facies, often accompanied by varying degrees of cyanosis. In cases with cardiac enlargement, the left anterior chest may bulge, the cardiac dullness border expands, and a systolic tremor caused by tricuspid regurgitation can be palpated at the left sternal border. The pulsations in the lower part of the apex and the apex area are normal or weakened. Due to significant enlargement of the right atrium and atrialized right ventricle, jugular venous pulsations are not prominent. On cardiac auscultation, heart sounds are faint, and a systolic murmur caused by tricuspid regurgitation can be heard at the left sternal border. Sometimes, a diastolic murmur caused by tricuspid stenosis may also be heard, with the murmur intensity increasing during inspiration. Due to delayed closure of the enlarged anterior tricuspid leaflet, the first heart sound is split, and the delayed component is enhanced. The second heart sound is also often split, and the pulmonary stirred pulse valve closure sound is faint. Some cases may exhibit a gallop rhythm. Abdominal examination may reveal an enlarged liver, but hepatic pulsations are rare. In childhood patients with severe cyanosis, clubbing of fingers (toes) may occur.
(1) X-ray findings Typical cases may show enlargement of the right atrium and upward and lateral displacement of the right ventricular outflow tract, narrowing of the superior mediastinum, and normal or reduced pulmonary vascular markings. A few cases may show no abnormal signs on cardiac imaging.
(2) Electrocardiogram findings The typical manifestations include right atrial hypertrophy with tall, peaked P waves, incomplete or complete right bundle branch block. Right axis deviation, low R-wave voltage in precordial leads, prolonged P-R interval, and frequent supraventricular arrhythmias are observed. About 5% of patients exhibit type B Wolff-Parkinson-White syndrome.
(3) Two-dimensional echocardiography and Doppler examination These reveal an enlarged anterior tricuspid leaflet with increased mobility. The septal and posterior leaflets are significantly displaced downward, hypoplastic, and exhibit poor mobility. Delayed tricuspid valve closure, leftward displacement of the valve membrane, and paradoxical motion of the interventricular septum are observed. The right atrium and atrialized right ventricle together display a massive right atrial cavity, while the functional right ventricular cavity shows shortened longitudinal dimensions. Doppler examination may demonstrate right-to-left shunting at the atrial level and tricuspid regurgitation.
(4) Right heart catheterization and selective angiography The right atrial cavity is markedly enlarged with elevated pressure, and the pressure curve shows tall a and V waves. The atrialized right ventricle exhibits atrial pressure curves, while the intracavitary electrocardiogram shows right ventricular patterns. In cases with an atrial septal defect, the catheter may pass from the right atrium into the left atrium. Right-to-left shunting may be observed at the atrial level, with normal right ventricular systolic pressure but elevated end-diastolic pressure. Some cases may show a transvalvular pressure gradient across the tricuspid valve. Right heart angiography reveals significant enlargement of the right atrium occupying the left ventricular position, with the functional right ventricle located in the right ventricular outflow tract. The valve membrane orifice is displaced to the left of the spine, and the inferior border of the right ventricle may show a notch at the tricuspid annulus and another notch between the atrialized ventricle and the functional ventricle. The pulmonary trunk and its branches appear thin. If right-to-left shunting is present at the atrial level, the left atrium will opacity prematurely.
bubble_chart Treatment Measures
The prognosis of Ebstein's anomaly varies significantly. Clinically, approximately 80% of patients with grade III cyanosis die around the age of 10, while only 5% of those with grade I cyanosis die at the same age. Most patients die within 2 years after the onset of congestive heart failure, and about 3% of cases experience sudden death. Common causes of death include congestive heart failure, arrhythmia, hypoxia, or pulmonary infection. Adult patients often die from recurrent thromboembolism, cerebrovascular accidents, or brain abscesses. Most patients die before the age of 20, with an average age of death being 20.
(1) Indications for Surgical Treatment Patients present with symptoms such as lack of strength, palpitations, shortness of breath, arrhythmia, cyanosis, and heart failure. In cases without right-to-left shunting at the atrial level, right heart failure is particularly severe and refractory. In contrast, in cases with a patent foramen ovale or atrial septal defect, significant cyanosis occurs due to right-to-left shunting, while symptoms of right heart failure are milder, though physical activity remains severely limited. Patients with right heart failure or cyanosis are candidates for surgery, and surgical treatment should be performed once the diagnosis is confirmed.
(2) Preoperative Preparation and Postoperative Management
1. Preoperative treatment with cardiotonic and diuretic drugs to alleviate symptoms of right heart failure, such as hepatomegaly and ascites.
2. Hepatomegaly and congestion may lead to impaired liver function and prolonged prothrombin time. Preoperative treatment with vitamin K and prothrombin complex is necessary.
(3) Surgical Methods (Three Approaches)
1. **Glenn Procedure** This involves an anastomosis between the superior vena cava and the right pulmonary artery. It reduces right heart load, decreases right-to-left shunting, increases pulmonary artery oxygen content, improves symptoms, and alleviates cyanosis. However, it is a palliative procedure that does not correct the anomaly and is typically used for infants with severe cyanosis who are unsuitable for definitive surgery. The short-term outcomes are acceptable, but the long-term results are poor, with patients often dying from arrhythmias.
2. **Valve Replacement** Under cardiopulmonary bypass, the right atrium is incised, and the tricuspid valve, chordae tendineae, and papillary muscles are excised. An appropriate artificial valve is selected. To avoid injury to the cardiac conduction system, the prosthetic valve is sutured anteriorly to the atrioventricular ring and posteriorly to the right atrial septum a few millimeters cephalad to the coronary sinus orifice, relocating the coronary sinus into the ventricular cavity. Any associated atrial septal defect is closed simultaneously. In Ebstein’s anomaly, the right atrium is extremely dilated, leading to slow blood flow and a high risk of thrombosis. For tricuspid valve replacement, bioprosthetic valves, with their central flow characteristics, offer superior hemodynamic performance compared to mechanical valves. Additionally, since right ventricular systolic pressure is not high, bioprosthetic valves in the tricuspid position have a significantly longer lifespan than those in the left heart, making them the preferred choice for valve replacement (Figure 1).
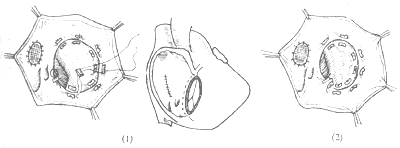
(1) Suture method for annuloplasty (2) After annuloplasty, the anterior leaflet closes the right atrioventricular orifice
Figure 1: Artificial Valve Replacement Surgery
3. Valve membrane plasty: Multiple mattress sutures with Teflon felt are used to fix the displaced septal leaflet and the root attachment area of the posterior leaflet to the tricuspid annulus, thereby reducing the enlarged annulus and eliminating the atrialized ventricle. To avoid conduction block, posterior leaflet plasty can be performed. Using double-armed sutures with pledgets, two parallel rows of sutures are placed from the septal-posterior commissure to the posterior-anterior commissure, spaced 3–4 mm apart, and then tied after pledgeted plication to reduce the tricuspid annulus, with a two-finger width as the objective standard. The normally developed anterior leaflet is utilized to restore the tricuspid valve's closing function. This procedure preserves the natural valve membrane and eliminates the potential risks of an artificial valve membrane, but it is only applicable to cases with a normally developed tricuspid anterior leaflet. Postoperatively, some degree of regurgitation may persist (Figure 2). Active medical treatment is still required postoperatively to control heart failure and arrhythmias, with close monitoring of serum potassium, sodium, chloride levels, and ECG changes, supplemented by timely potassium chloride. Postoperative care includes maintaining drainage tube patency and hemostasis measures such as fibrinogen infusion or fresh blood transfusion. Postoperatively, cyanosis disappears, the liver shrinks, ascites resolves, and the cardiac shadow significantly reduces, with satisfactory outcomes.
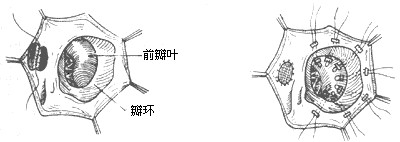
(1) Intracardiac displacement deformity (2) The displaced leaflet is sutured to the annulus to eliminate the atrialized right ventricle
Figure 2 Valve membrane plasty






