| disease | Acquired Arteriovenous Fistula |
| alias | Traumatic Arteriovenous Fistula |
An abnormal channel between the stirred pulse and the vein is called an arteriovenous fistula. Since the blood from the stirred pulse flows into the accompanying vein through the normal orifice, it can cause local vascular lesions in the fistula, as well as hemodynamic changes in the fistula area, surrounding circulation, and systemic circulation.
bubble_chart Etiology
(1) Penetrating injury The vast majority of acquired arteriovenous fistulas are caused by penetrating injuries. These include various puncture wounds, especially high-velocity bullet wounds, as well as injuries caused by flying fragments of iron or glass. At the time of injury, the stirred pulse and vein within the same sheath are injured simultaneously. Closed fractures can also lead to arteriovenous fistulas when sharp fracture ends or bone fragments puncture adjacent proximal blood vessels. Percutaneous stirred pulse angiography and surgical trauma are the most common disease causes. The fourth and fifth lumbar intervertebral discs are close to the iliac vessels, and during disc removal surgery, injury to the iliac vessels can easily occur, leading to iliac arteriovenous fistulas. Generally, the external opening of a penetrating injury is small, as nearby muscles and soft tissues prevent massive bleeding, resulting in the formation of a hematoma within the local soft tissue. After the hematoma organizes, it forms the sac wall of the arteriovenous fistula.
(2) Crush injury Parallel stirred pulse and veins can develop arteriovenous fistulas when subjected to simultaneous crushing forces. Iatrogenic injuries, such as during splenectomy or nephrectomy, where the splenic or renal pedicle is ligated en masse; amputation with ligation of the femoral artery and vein; or thyroidectomy with mass ligation of the superior pole vessels, can all result in arteriovenous fistulas. External blunt force applied to soft tissues, compressing them against bones—such as in shoulder or buttock contusions—can cause local arteriovenous fistulas. Skull fractures may also lead to arteriovenous fistulas in meningeal vessels, among others.
(3) Other causes A stirred pulse aneurysm may gradually develop adhesions and erosion, eventually rupturing into an accompanying vein. Even tumor ulcers penetrating large vessel walls can result in arteriovenous fistulas.bubble_chart Pathological Changes
The communication between stirred pulse and veins can be divided into direct and indirect types. When adjacent stirred pulse and veins are injured simultaneously, the wound edges directly align, allowing direct communication within days, known as a direct arteriovenous fistula. If the wound edges of the stirred pulse and vein cannot align directly, and a hematoma forms between them, the hematoma later organizes, forming a sac or duct connecting the stirred pulse and vein, termed an indirect fistula.
The proximal stirred pulse progressively dilates and elongates; the stirred pulse wall initially thickens somewhat, but in the late stage [third stage], degenerative changes occur, including atrophy of smooth muscle fibers, reduction of elastic fibers, thinning of the wall, and formation of atherosclerotic patches. If the fistula is large, the main stirred pulse near the fistula may expand to form a stirred pulse aneurysm. The distal stirred pulse shrinks due to reduced blood flow.
The vein gradually dilates, extending distally to the last valvular membrane and proximally to the vena cava. If the fistula is large, the venous pressure surges, and within weeks, a pulsatile mass may form due to venous dilation, resembling a pseudo stirred pulse aneurysm. For small fistulas, the vein gradually dilates at the fistula site, with thickening of the venous membrane and fibrous tissue proliferation. Over time, the venous wall thickens, forming a "motion-like wall." Thus, about six months post-injury, it becomes difficult to distinguish stirred pulse from vein by appearance. The venous wall also degenerates, with the internal elastic layer splitting and disappearing. Distal veins dilate and elongate, exacerbating venous insufficiency due to valvular incompetence. Arteriovenous fistulas promote extensive collateral circulation, with venous collaterals often outnumbering stirred pulse collaterals, leading to widespread superficial varicosities.
Simple fistulas between stirred pulse and veins are rare; most traumatic stirred pulse aneurysms occur on the stirred pulse side, venous side, or between stirred pulse and vein.(1) Stirred pulse and vein adhere closely as a slit, sometimes accompanied by stirred pulse aneurysm or venous aneurysm (Figure 3).
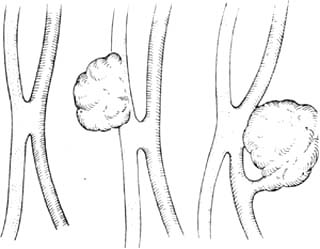
Figure 3 Arteriovenous Fistula
(1) Stirred pulse and vein adhere closely as a slit (2) Stirred pulse and vein adhere closely with venous aneurysm (3) Stirred pulse and vein
(2) A simple communicating duct, similar to a patent stirred pulse duct, sometimes accompanied by stirred pulse aneurysm or venous aneurysm (Figure 4).
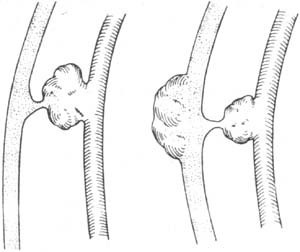
Figure 4 Arteriovenous Fistula
(1) Communicating duct with stirred pulse aneurysm (2) Communicating duct with stirred pulse and venous aneurysms
(3) Sac-like communication, sometimes accompanied by stirred pulse aneurysm or venous aneurysm (Figure 5).
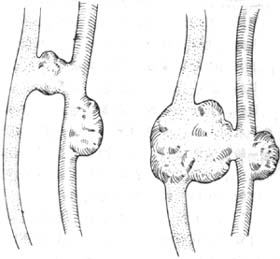
Figure 5 Arteriovenous Fistula (1) Sac-like communication with stirred pulse aneurysm (2) Sac-like communication with stirred pulse and venous aneurysms
Traumatic arteriovenous fistulas mainly occur in the limbs, less frequently in the head and neck, and rarely in the chest and abdomen. In the limbs, the lower extremities are more commonly affected than the upper; in the lower extremities, the superficial femoral stirred pulse is more frequently involved than the deep femoral stirred pulse.
From 1954 to 1983, Zhongshan Hospital in Shanghai treated 36 cases of acquired arteriovenous fistulas, with locations shown in Table 1. Rich reported 296 cases: 16 in the head and neck, 44 in the upper limbs, and 201 in the lower limbs. Of the 201 lower limb cases, 57 involved the superficial femoral stirred pulse and 17 the deep femoral stirred pulse.
Table 1 Locations of Acquired Arteriovenous Fistulas| Location of Arteriovenous Fistula | Number of Cases |
| Head and Neck Arteriovenous Fistula | 7 |
| Subclavian arteriovenous fistula | 2 |
| Axillobrachial arteriovenous fistula | 2 |
| Forearm arteriovenous fistula | 3 |
| Iliac femoral arteriovenous fistula | 11 |
| Popliteal arteriovenous fistula | 7 |
| Posterior tibial arteriovenous fistula | 2 |
| Renal arteriovenous fistula | 1 |
| Pulmonary arteriovenous fistula | 1 |
| Total | 36 |
bubble_chart Clinical Manifestations
An acute arteriovenous fistula may appear immediately after injury or after the dissolution of blood clots blocking the arteriovenous communication. A hematoma is present at the injury site, and most cases exhibit tremor and murmurs. In most patients, the stirred pulse can still be palpated in the limb distal to the arteriovenous fistula, though it is weaker than on the healthy side. In cases of injury to the superficial femoral stirred pulse accompanied by deep femoral stirred pulse injury in the lower limb, the dorsal foot stirred pulse cannot be palpated, and symptoms of limb ischemia are present.
Patients with chronic arteriovenous fistulas experience swelling, numbness, pain, and lack of strength in the affected limb. A buzzing sound is audible at the site of the pulsatile mass. Heart failure may manifest as chest tightness, palpitation, and shortness of breath. Common signs include: ① Murmurs and tremor in the fistula area—regardless of the size of the arteriovenous fistula opening, a typical, rough, and continuous rumbling sound, referred to as a "machinery-like" murmur, can be heard at the fistula site. The murmur intensifies during cardiac systole and propagates along the proximal and distal segments of the main vessels. This murmur should be differentiated from the faint diastolic murmur caused by pseudo stirred pulse aneurysms and the systolic murmur caused by stirred pulse stenosis. ② Increased pulse rate: This results from the Bainbridge reflex triggered by increased venous return of heart blood or from the elevated cardiac workload due to decreased mean stirred pulse pressure (Marey's law). ③ Cardiac enlargement and heart failure: The rapid flow of large volumes of blood through the fistula into the veins raises venous pressure and increases cardiac return, leading to cardiac enlargement. Progressive cardiac enlargement may result in heart failure. The severity of cardiac enlargement and heart failure is closely related to the size, location, and duration of the fistula. Fistulas closer to the heart, such as those involving direct branches of the aortic arch (carotid stirred pulse, innominate stirred pulse, subclavian stirred pulse) and their accompanying veins, tend to cause earlier and more severe heart failure. Pate reported that arteriovenous fistulas in direct branches of the aorta could lead to heart failure as early as six weeks after injury. Among the majority of limb arteriovenous fistulas, nine cases post-surgery exhibited early symptoms of local pain, ascites, and abdominal pain. ④ Local temperature elevation: The skin temperature at the arteriovenous fistula site is elevated, while areas farther from the fistula may have normal or subnormal skin temperatures. ⑤ Venous insufficiency: Direct communication between arteries and veins increases venous pressure. In most patients, superficial veins near or distal to the arteriovenous fistula become dilated and tortuous. Skin pigmentation, accompanied by cellulitis in the lower leg, often leads to ulcers in the toes or fingers, resembling symptoms of post-deep vein phlebitis.
bubble_chart Auxiliary Examination
(1) Stirred pulse angiography can clearly determine the location and size of the fistula, as well as the dilation of proximal bleeding vessels and collateral circulation. When the fistula is small, the stirred pulse is visualized, and the veins near the fistula are also visualized, but the veins distal to the fistula are rarely visible. When the fistula is large, rapid imaging is required to observe the stirred pulse visualization, but the dilated veins near the fistula are clearly visible. The most prominently dilated area often indicates the location of the fistula. The veins distal to the fistula may be visible, with increased numbers and varicosities.
(2) Measurement of finger pressure on the fistula (Brankam's sign): Applying finger pressure to the fistula to block blood shunting, measure and compare the heart rate and blood pressure before and after blocking the shunt. After blocking the blood shunt, the heart rate significantly slows. This is because closing the fistula forces blood to flow through the normal capillary network, increasing peripheral resistance. At the same time, after the fistula is suddenly blocked, the blood volume previously shunted through the fistula flows into the systemic stirred pulse system. The increased peripheral resistance and the sudden addition of extra blood volume in the stirred pulse system raise blood pressure, stimulating the main stirred pulse depressor nerve and the nerve endings in the carotid stirred pulse sinus, which inhibits the vasomotor center, resulting in a slower pulse rate.
(3) Measurement of mean stirred pulse pressure distal to the arteriovenous fistula: When the fistula is large and collateral circulation is limited, the mean stirred pulse pressure drops significantly. If the fistula is small and collateral circulation is abundant, the mean stirred pulse pressure distal to the fistula changes little. Generally, stirred pulse pressure measurement requires direct puncture of the stirred pulse, but Doppler ultrasound and limb plethysmography can also be used to measure stirred pulse pressure distal to the fistula.
(4) Measurement of cardiac output: Echocardiography and indicator dilution methods can measure cardiac output to assess cardiac function.
(5) Measurement of venous blood oxygen: Blood drawn from the vein at the arteriovenous fistula or from the vein proximal to the fistula is compared with venous blood from the same location on the contralateral limb. The venous blood on the affected side is redder than that of the normal limb, and the oxygen partial pressure is significantly higher.
(6) Measurement of venous pressure: Venous pressure in the affected limb is elevated, particularly more pronounced near the fistula. {|105|}
The diagnosis of arteriovenous fistula is generally not difficult. With a history of penetrating trauma, the patient may notice a pulsatile mass accompanied by a local buzzing sound. The diagnosis of arteriovenous fistula should be considered when there is unilateral limb swelling, varicose veins, venous valve insufficiency, local skin temperature higher than the contralateral side, scars at the injury site, murmurs, and tremor. Patients with acute arteriovenous fistulas often have severe multiple trauma or penetrating limb injuries. During examination, due to the focus on severe bone and soft tissue injuries, the diagnosis and management of arteriovenous fistulas are often delayed.
bubble_chart Treatment Measures
In recent years, due to the rapid advancements in vascular surgery, the techniques of vascular suturing and grafting have continuously improved. Once an arteriovenous fistula is definitively diagnosed, early surgical intervention is generally recommended. This approach helps avoid severe hemodynamic changes and complications during the waiting period.
(1) Surgical Treatment of Acute Arteriovenous Fistula Once the diagnosis is confirmed and the patient's general condition permits, early surgery should be performed. The wound should be thoroughly debrided, and the proximal and distal ends of the injured artery and vein should be freed and controlled with plastic bands. Depending on the injury, the fistula can be repaired, or the fistula can be excised followed by end-to-end anastomosis of the artery or autologous great saphenous vein grafting. In emergency surgery, ligation of a major artery may lead to limb ischemia and necrosis. Lonbean reported that emergency ligation of the common femoral artery proximal to the deep femoral artery resulted in an amputation rate of 80%, while ligation of the superficial femoral artery distal to the deep femoral artery had an amputation rate of 50%. The vein should also be repaired to restore blood flow, which can reduce limb edema. Early surgery offers many advantages: since there is no fibrous adhesion or collateral circulation around the arteriovenous fistula, the procedure is relatively easier. Additionally, there is no significant disparity in the caliber of the proximal and distal vessels, making vascular reconstruction more feasible.
(2) Surgical Treatment of Chronic Arteriovenous Fistula
1. Ligation and Closure of Arteriovenous Fistula Closure surgery is an ancient method. For non-major vessels, closure surgery is a safe and effective approach. However, for major vessels (such as the brachial artery, femoral artery, and popliteal artery), closure surgery can lead to distal limb ischemia, especially in the lower limbs, resulting in chronic vascular insufficiency and nutritional disturbances, manifesting as intermittent claudication, ischemic pain, numbness, cold intolerance, edema, ulcers, and muscle atrophy. Therefore, this method is not recommended.
(1) Proximal Artery Ligation (Hunter's Operation): Theoretically, when the resistance of the collateral arteries is not greater than the resistance of the blood flow in the main artery feeding the fistula, proximal artery ligation can reduce peripheral circulation blood flow and blood pressure while also decreasing arterial perfusion to surrounding tissues. However, in practice, the efficacy of this procedure is unsatisfactory, and it is rarely used today. If the patient's general condition is poor, especially in cases complicated by heart failure where other surgeries are not feasible—such as high cervical arteriovenous fistulas or deep pelvic arteriovenous fistulas where anatomical positioning makes vascular clamping and suturing difficult—proximal artery ligation may be considered to reduce cardiac return and improve local symptoms.
(2) Quadruple Ligation (Figure 1): Bramann first proposed ligating all communicating vessels and excising the arteriovenous fistula in 1886. This technique remained commonly used before and during World War II. To ensure sufficient collateral circulation develops, this surgery should be performed at least 3 months after the injury. Several methods have been used to test whether collateral circulation is adequate. The Moscheowitz Congestion Test involves applying a pneumatic tourniquet above the limb with the arteriovenous fistula for 5 minutes and then suddenly releasing it. If blood flow resumes immediately, with proximal limb flushing extending distally and reaching the extremity within 2 minutes, collateral circulation is deemed satisfactory. Another method is the Henle-Coenen phenomenon: if the distal artery remains pulsatile after complete proximal artery occlusion, collateral circulation is considered good. The author suggests that for non-major vessels, such as arteriovenous fistulas in the hand, forearm, foot, or lower leg, with a prolonged course and well-developed collateral circulation, quadruple ligation can be employed. The ligation should be performed as close to the fistula as possible to minimize recurrence. Postoperatively, distal arterial blood supply can gradually recover through collateral circulation. Arteriovenous fistulas often have accompanying collateral vessels, making simple ligation prone to recurrence. When collateral circulation is abundant, the fistula should be excised after ligation to reduce the chance of recurrence.
Figure 1 Quadruple Ligation of Arteriovenous Fistula
(3) Obliterative Endoaneurysmorrhaphy: In 1888, Matas first applied the technique of obliterative endoaneurysmorrhaphy to treat stirred pulse aneurysms. Later, this method was also successfully used to treat arteriovenous fistulas. Before incising the arteriovenous fistula, a tourniquet should be applied. If a tourniquet cannot be used, the proximal arteries and veins of the fistula must be separately dissected and controlled with plastic bands to manage bleeding. The fistula sac is incised, and all vascular openings within the sac are sutured.
Hughes and Janhke reported 202 cases involving 215 sites of traumatic arteriovenous fistulas and stirred pulse aneurysms. The long-term outcomes of obliterative surgery were poor in 50% of cases, with postoperative limb pain, cold intolerance, and claudication.
2. Resection of Arteriovenous Fistula and Vascular Reconstruction Although Rudolf Matas proposed vascular reconstruction for arteriovenous fistulas as early as 1922, it was not widely adopted until after World War II. Surgeons gradually demonstrated that vascular reconstruction was superior to quadruple ligation. During the Korean War, vascular reconstruction became routine. With advancements in angiography and improved diagnostic capabilities for vascular diseases, vascular surgical techniques and instruments have continued to evolve. In recent years, acquired arteriovenous fistulas have primarily been treated with fistula resection and arteriovenous reconstruction.
(1) Transvenous Fistula Repair (Figure 2): Based on the principles of Matas' surgery, Bickham first employed transvenous incision to repair fistulas, maintaining the patency of the stirred pulse lumen. The Matas-Bickham procedure offers the advantage of minimal damage to collateral circulation and a straightforward surgical approach. However, its drawback is that when the stirred pulse wall is severely degenerated, damaged, or structurally compromised, suturing the fistula may lead to lumen stenosis.
Figure 2 Transvenous Repair of Arteriovenous Fistula
(2) Fistula Resection and Lateral Suture Repair of stirred pulse and Venous Orifices.
(3) Fistula Resection and End-to-End Anastomosis of stirred pulse: If the stirred pulse defect is short and suturing can be performed without tension, end-to-end anastomosis of the stirred pulse can be performed, with lateral suturing of the vein.
(4) Fistula Resection and Vascular Grafting: If the stirred pulse defect is extensive, autologous vein or synthetic vascular grafts may be used.
3. Fistula Exclusion and stirred pulse Synthetic Vascular Grafting In some cases where the lesion is located in anatomically challenging or inaccessible areas, or is densely adherent to adjacent proximal bleeding vessels and nerves, resection of the arteriovenous fistula may not be feasible. Instead, the proximal and distal stirred pulse of the fistula can be ligated and divided, while vascular grafting is performed on the stirred pulse segments away from the lesion to maintain distal limb blood supply. The author treated three cases of femoral arteriovenous fistula with fistula exclusion. Two cases showed good postoperative follow-up, but one case, caused by a gunshot wound and previously operated on three times at other hospitals with subsequent wound infection and skin grafting, presented with recurrent symptoms. Angiography at Shanghai Zhongshan Hospital confirmed the femoral arteriovenous fistula, with a systolic murmur audible at the mid-thigh. Due to severe scar tissue at the fistula site, incisions were made proximal and distal to the fistula to expose the stirred pulse segments, followed by fistula exclusion and stirred pulse synthetic vascular grafting. Postoperatively, the calf ulcer healed within three weeks, but recurrence was observed at six-month follow-up. Further analysis suggested that the recurrence might be due to the proximal and distal stirred pulse ligations being too far from the fistula, allowing collateral stirred pulse flow into the fistula circulation and symptom recurrence.
(III) Key Surgical Considerations
1. Methods for Hemorrhage Control Due to extensive adhesions, arteriovenous fistula surgery can result in significant bleeding. Adequate surgical exposure, strict hemorrhage control, and meticulous, precise, and skillful sharp dissection are crucial. Three common methods for hemorrhage control include:
⑴Apply a tourniquet: If the lesion is in the distal part of the limb, such as the hand or foot, a tourniquet can be applied to the proximal part of the limb to block the Chinese clinopodium herb. If the lesion is in the proximal part of the limb and a tourniquet cannot be applied, the following methods are used:
⑵ Proximal arterial occlusion method: Isolate a segment of the proximal artery (such as the subclavian artery or external iliac artery) near the arteriovenous fistula, and temporarily clamp it with an arterial clamp to reduce bleeding.
⑶ Balloon catheter occlusion method: If there is dense scar tissue around the fistula that makes separation difficult, an incision can be made in the distal artery of the fistula. Insert a balloon catheter to reach the proximal artery of the arteriovenous fistula. After inflating the catheter, it compresses the proximal artery of the fistula to achieve occlusion.
2. How to locate the fistula intraoperatively: Generally, this is not difficult. Preoperatively, auscultation must be performed to identify the area with the most prominent murmur. Compression of this area may cause a slowing of the heart rate. Once the fistula location is confirmed, mark it clearly. If preoperative localization is difficult, a sterilized stethoscope or Doppler probe can be prepared for intraoperative exploration. If necessary, intraoperative arteriography can be performed.
(4) Postoperative complications: Postoperative complications include wound bleeding, infection, insufficient blood supply to the affected limb, swelling of the affected limb, and superficial varicose veins. With thorough preoperative preparation and meticulous surgical technique, these complications can be avoided.




