| disease | Renovascular Hypertension |
| alias | Renal Hypertension |
There are many causes of hypertension, and that caused by kidney disease is called renal hypertension. Renal hypertension can be divided into two categories: one is kidney parenchymal disease, and the other is renal vascular disease.
bubble_chart Epidemiology
General literature indicates that renovascular hypertension accounts for approximately 5–10% of all hypertension cases. In our country, there are no precise statistics available. Reports suggest that around 800,000 young adults in China suffer from this condition. Shanghai Zhongshan Hospital analyzed 3,145 cases of ten common kidney diseases over a 25-year period from 1950 to 1975, identifying 655 cases complicated by hypertension, accounting for 20.8% of the total kidney disease cases. This included 62 cases of renal artery stenosis, indicating that renovascular hypertension constituted 9.04% of renal hypertension. During the same period, the hospital admitted a total of 3,365 hypertension patients, meaning renal hypertension accounted for 18.5% of hospitalized hypertension cases, while renovascular hypertension represented approximately 1.88% of all hypertension cases. Since the hospital had only been conducting clinical work on renovascular hypertension for slightly over a decade at that time, the actual number of cases is likely higher than the reported statistics.
Multiple factors influence the statistical incidence of renovascular hypertension, such as specialized centers reporting higher rates compared to general or comprehensive medical institutions. The duration of the disease and the severity of blood pressure are also related. In the early stages, it is often functional, with fluctuating blood pressure. When renin secretion increases, blood pressure rises, but the body's regulatory mechanisms can lower it, making it difficult to distinguish from general hypertension. As vascular lesions progress and blood pressure remains persistently elevated, the diagnosis becomes easier to confirm. Dean (1984) emphasized that the incidence of this disease is related to blood pressure severity. Citing Hollifield's analysis of 137 hypertension cases, he noted that if diastolic blood pressure was between 12.7–15.3 kPa (95–115 mmHg), renovascular hypertension was rare, but when it exceeded 15.7 kPa (118 mmHg), the incidence could reach 26%. Davis also pointed out that renovascular hypertension accounts for about 31% of malignant hypertension cases.
bubble_chart PathogenesisIn 1906, Janeway narrowed the renal stirred pulse on one side of a dog, resulting in hypertension that lasted for 105 days, but no further research was conducted. It wasn't until 1934, when Goldblatt's experiment created an animal model of renal ischemic hypertension, that the condition regained attention, thereby laying the theoretical foundation for renovascular hypertension.
The mechanism by which renal stirred pulse narrowing causes hypertension is not yet fully understood. It is generally believed that the reduction in renal blood flow leading to renal ischemia is a contributing factor. However, some researchers, using direct measurements of renal blood flow before and after narrowing the stirred pulse in the dog's testis and penis, found that in cases of grade I or mild renal stirred pulse narrowing-induced hypertension, the reduction in renal blood flow was only temporary. With the establishment of collateral circulation, renal blood flow could return to normal levels. Others found that making animals breathe oxygen-deficient gases or perfusing the kidneys with venous blood did not produce hypertension. Therefore, while renal ischemia and hypoxia are factors contributing to renovascular hypertension, other conditions must also be present.
Regarding the pathogenesis of renovascular hypertension, there are multiple theories in the literature. Currently, the three most widely accepted are as follows:
1. **The renal pressor system—the renin-angiotensin-aldosterone system (RAA system)** Renin is a proteolytic enzyme with heat-labile and non-dialyzable properties. Renin itself is not a pressor substance; it must combine with α2
In addition to producing renin, the kidneys also produce hypertension protease, which has the ability to break down angiotensin. Under normal conditions, these two systems remain balanced and do not cause hypertension. However, in cases of renal ischemia or hypoxia, increased renin secretion disrupts this balance, leading to excessive angiotensin production and resulting in hypertension.
Experiments have shown that the kidneys can influence aldosterone secretion, and both angiotensin II and III can stimulate aldosterone secretion. This indicates that the hypertension caused by angiotensin II and III involves two mechanisms: on one hand, constriction of small stirred pulses increases peripheral resistance; on the other hand, increased aldosterone secretion promotes sodium and water retention, expanding extracellular fluid volume. Thus, the renin-angiotensin-aldosterone system is formed.Vander (1984) provided a comprehensive explanation of the role of the renin-angiotensin-aldosterone system (Figure 9).
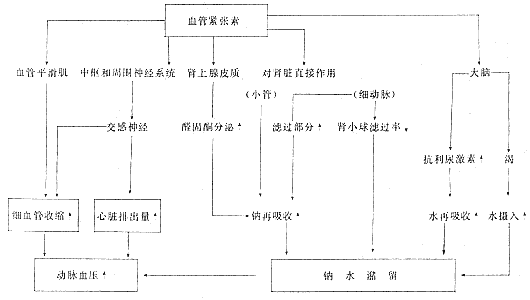
II. The Kidney's System for Regulating Hypertensive Substances Recent experiments have demonstrated that, in addition to the renin-angiotensin-aldosterone system mentioned above, there is another system for regulating blood pressure: the kallikrein-kinin-prostaglandin system (KKP system).
1. The Renal Kallikrein-Kinin System Kinins are formed when kininogen from the liver is acted upon by kallikrein produced by the kidneys. Over 90% of renal kallikrein is distributed in the cortex, with 4.5% in the medulla and 4.1% in the papilla. Within the cortex, the glomeruli contain only 1.5% of the active enzyme for kinins, while the primary site of production is likely the juxtaglomerular apparatus. The higher the activity of kallikrein, the more it catalyzes the hydrolysis of kininogen to produce kinins. Additionally, the kidneys secrete kinin hydrolase, which can degrade the generated kinins.
Currently, renal kinins are believed to have the following effects: ① Promoting the dilation of small arteries, thereby reducing peripheral vascular resistance; ② Dilating intrarenal small arteries, increasing renal blood flow, and improving cortical ischemia; ③ Promoting the excretion of sodium and water, with water excretion exceeding sodium excretion, leading to a decrease in urine osmotic pressure. Increased excretion of water and sodium results in reduced plasma volume, elevating hematocrit and total plasma protein concentration; ④ Due to decreased peripheral vascular resistance and reduced circulating blood volume, blood pressure may decrease, thus exerting an antihypertensive effect.
These effects are primarily mediated by kinins promoting the production of prostaglandins, though some are also direct actions of kinins.
2. Renal Prostaglandins Several prostaglandins have been identified to date, with three types isolatable from the renal medulla: PGE2, PGA2, and PGF2α. Prostaglandin levels are very low in the renal cortex, while the papilla of the medulla contains higher concentrations. Within the kidney, PGE2 is present in smaller amounts, with PGA2 and PGF2α being predominant. Prostaglandins are generally not stored within cells; once synthesized, they are immediately released. Some are transported via intrarenal circulation to the cortex to exert physiological effects, while another portion of PGE2 and PGF2α enters systemic circulation through the renal vein and is degraded by lung tissue. PGA2, however, can persist in systemic circulation.
The role of renal prostaglandins: ① Renal prostaglandin E2 can cause local vasodilation, increasing renal blood flow; ② Prostaglandins can also redistribute renal blood flow, reducing medullary blood flow while increasing cortical blood flow; ③ PGE2 and PGA2 can dilate the renal afferent arterioles, increasing the hydrostatic pressure in the peritubular capillaries surrounding the proximal tubules, thereby reducing the reabsorption of water and salts by the proximal tubules. PGA2 and PGE2 can inhibit the activity of Na+-K--ATPase on the renal tubular cell membrane, making it harder for intracellular Na+ to be transported into the peritubular fluid, thereby impairing the reabsorption of sodium and water by the renal tubules and producing a diuretic effect. PGE2 can also inhibit antidiuretic hormone, increasing urine output and promoting the excretion of sodium, potassium, and water. Renal prostaglandins also antagonize the effects of catecholamines, but do not inhibit catecholamine secretion. Therefore, the renin-prostaglandin system plays a role in modulating elevated renin levels. This is because angiotensin stimulates increased aldosterone secretion, which in turn increases the secretion of prostaglandin-releasing enzymes, thereby accelerating the synthesis of prostaglandins PGA2 and PGE2.
3. Renoprival Hypertension Renoprival hypertension refers to hypertension caused by the absence of functional renal tissue, also known as renal absence hypertension or post-nephrectomy hypertension. In addition to producing antihypertensive substances, the kidneys also regulate body fluids and electrolytes and excrete pressor factors from the body. Clinically, hypertensive patients excrete more water and sodium than normal individuals, with water excretion being relatively greater, leading to an increased proportion of sodium in the body. On the other hand, hypertension generally occurs when body fluid volume increases, and dehydration can lower blood pressure, while large-volume fluid infusion can raise it. This phenomenon is particularly evident in uremic hypertension. Such patients are more sensitive to sodium and water retention, and animal experiments have confirmed similar findings. A significant reduction in renal tissue can make animals highly sensitive to a high-salt diet; after bilateral nephrectomy, high salt intake can induce renoprival hypertension. Therefore, the complete loss of renal function, as in bilateral nephrectomy, results in hypertension significantly related to imbalances in body fluids and sodium salt balance. Additionally, pressor substances in the body cannot be excreted after renal removal, leading to elevated blood pressure due to abdominal mass.
Combining the above three theories, the three types of peripheral vascular fluid renin values observed in clinical cases of renovascular hypertension can be explained as follows.
1. High-Renin Hypertension (Vasoconstrictive Hypertension) An animal model can be created to demonstrate this type. Constricting one renal artery while keeping the other normal reduces blood supply and intrarenal pressure in the stenotic kidney, stimulating increased renin secretion, which raises angiotensin levels and induces hypertension. The contralateral healthy kidney, subjected to hypertensive stress, shows decreased renin secretion. The increase in renin from the diseased kidney outweighs the decrease from the healthy kidney, resulting in plasma renin levels higher than normal, forming high-renin hypertension. Treatment involves anti-renin medications.
2. Low-Renin Hypertension (Volume-Dependent Hypertension) This animal model involves constricting one renal artery and removing the contralateral kidney. With only a diseased kidney remaining, sodium and water excretion decrease, and sodium retention expands extracellular fluid volume (i.e., blood volume), leading to hypertension. Intrarenal pressure does not fall below baseline, so renin secretion does not increase. Under conditions of increased blood volume, plasma renin levels are correspondingly lower than normal, forming low-renin hypertension. Treatment does not involve anti-renin drugs but rather diuretics to promote sodium excretion.
3. Normal-Renin Hypertension (Mixed Hypertension) This type is common in primary hypertension. The kidneys in such cases exhibit both impaired sodium excretion and increased renin secretion—i.e., increased blood volume on one hand and enhanced vasoconstriction on the other, both contributing to elevated blood pressure. The rise in blood pressure and blood volume can suppress renin secretion, eventually reaching equilibrium, where renin, angiotensin, and aldosterone secretion remain within normal ranges. Treatment requires a combination of diuretics to promote sodium excretion and anti-renin medications.
bubble_chart Pathological Changes
The pathological changes of renovascular hypertension can be discussed from three aspects.
(1) Pathological changes in the kidneys: The pathological changes in the kidneys due to kidney excess vary depending on the underlying causes, primarily reflecting the pathological characteristics of the disease itself, such as glomerulonephritis, renal tumors, renal subcutaneous nodules, etc. However, there may also be corresponding changes in renal tissue due to secondary hypertension. In renovascular hypertension, the main lesions occur in the blood vessels and can be divided into acute and chronic types:
1. Acute changes: These mainly involve hyperplasia in the walls of the interlobar stirred pulse and intrarenal small stirred pulse (cellular hyperplasia in young individuals and elastic fiber hyperplasia in the elderly), leading to narrowing of the lumen. The walls of the stirred pulse and surrounding areas exhibit localized necrotic zones containing large amounts of fibrin, termed fibrinoid necrosis. Subendothelial hyperplasia results in the obstruction of fine stirred pulses, eventually leading to glomerular atrophy replaced by collagen.
2. Chronic changes: These are seen in long-term persistent hypertension. The fine stirred pulses in the kidneys, particularly the afferent stirred pulses, undergo sclerosis, causing ischemia in the nephrons and subsequent atrophy. As the condition worsens, fine stirred pulse-induced renal sclerosis may develop, with narrowed or occluded lumens. The kidneys shrink, harden, and become uneven in surface texture, scattered with fine granular protrusions and accompanied by small cystic cavities, forming a fine granular kidney.
(2) Pathological changes in the juxtaglomerular apparatus: Since the role of renin as a hypertensive factor was established, many researchers have focused on studying the production of renin in Unprocessed Rehmannia Root. Following Goormaghtigh (1944), Peart, Bing, Cook, and others, using techniques such as thick-sectioning, microdissection, and magnetic iron, discovered that renin is present in the tissues near the glomeruli, known as the juxtaglomerular apparatus (Figure 10). It consists of four components: ① Juxtaglomerular cells: responsible for renin production; ② Macula densa: a sensory body, where high sodium levels stimulate juxtaglomerular cells to increase renin secretion; ③ Goormaghtigh cells: a type of nerve terminal corpuscle with a regulatory function over renal secretion; ④ Becher cell clusters: involved in the absorption and synthesis of glucose, phosphates, and amino acids.
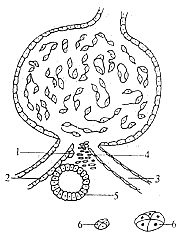
Figure 10: Schematic diagram of the juxtaglomerular apparatus
⒈ Juxtaglomerular cells; ⒉ Afferent stirred pulse; ⒊ Efferent stirred pulse; ⒋ Goormaghtigh cells; ⒌ Macula densa; ⒍ Becher cell clusters
In renovascular hypertension, the juxtaglomerular cells undergo a series of pathological changes, summarized as follows:
Renal stirred pulse stenosis → renal ischemia → decreased intrarenal pressure → increased number of juxtaglomerular cells → increased intracellular granules → increased renin secretion → elevated systemic blood pressure → increased intrarenal pressure in the contralateral kidney → decreased juxtaglomerular cells → decreased intracellular granules. This pathological process explains the theoretical basis for elevated renin in the affected kidney and reduced renin in the contralateral kidney in unilateral renal stirred pulse stenosis.
(3) Pathological changes in the renal blood vessels: In renovascular hypertension, the pathological changes in renal vascular diseases can be classified into several types. According to foreign literature, they are mainly divided into stirred pulse atherosclerosis and fibromuscular hyperplasia, each with distinct clinical features, with the former having a higher incidence than the latter. In China, pan-aortitis (multiple large stirred pulse inflammation) is the most commonly observed.
1. Atherosclerosis generally occurs in elderly patients, with a higher incidence in males than females. The resulting renal artery obstruction mostly occurs within the proximal 2cm, occasionally affecting the distal artery or its branches. The lesion originates in the intima, forming atherosclerotic plaques that spread along the vascular wall, leading to luminal stenosis and intimal damage. The intima is replaced by a mass of acellular atheromatous material, containing lipids, calcium deposits, necrotic debris-engulfing tissues, and thrombi. Renal artery atherosclerosis is often a local manifestation of systemic vascular disease. In unilateral cases, the left side is more frequently affected than the right.
2. Fibromuscular hyperplasia Commonly seen in young patients, with a higher incidence in females than males. The lesions mainly occur in the middle and distal thirds of the renal artery, often involving its branches, and unilateral cases are more frequently observed on the right side. The pathological changes in this type can be further divided into four subtypes: ① Intimal fibroplasia: The intima is significantly thickened with collagen accumulation, scattered primitive fibroblasts, and accompanying hematoma, which deforms the narrowed segment of the artery and shows a tendency to progress. Angiography reveals focal stenosis in the middle segment of the renal artery. ② Fibromuscular hyperplasia: The lesion occurs in the vascular media, with simultaneous hyperplasia of smooth muscle and fibrous tissue. The arterial wall shows concentric thickening, and elastic rupture leads to intramural hematoma, surrounded by abundant collagen formation. Angiography demonstrates smooth stenosis in the renal artery or its branches. ③ Medial fibroplasia: Mainly characterized by fibrous tissue hyperplasia, thinning or disappearance of the internal elastic membrane, replacement of muscle fibers by collagen, thinning of the media, and segmental aneurysmal dilation. The lesions are generally extensive, often extending to the distal two-thirds of the vessel or involving its branches. Angiography shows a beaded appearance of the renal artery. ④ Subadventitial fibroplasia: The lesion is located in the external elastic layer of the vessel, with collagen deposition in the outer membrane of the media. The renal artery is narrowed due to being surrounded by dense collagen. Angiography reveals irregular stenosis with abundant collateral circulation.
Among cases of renovascular hypertension, in addition to the above, a few are caused by renal artery aneurysm, renal infarction, renal arteriovenous fistula, renal arteritis, etc.
3. Takayasu arteritis (Multiple large arteritis) As early as the late 19th century, Savory and, in the early 20th century, Takayasu observed that primary arteritis of the thoracic aorta could occlude the large branches of the aortic arch, thereby affecting blood supply to the brain, eyes, and upper limbs. Later, it was also discovered that this inflammatory change occurs in the abdominal aorta and its branches. When the lesion involves the orifice of the renal artery, renovascular hypertension develops. This condition is most common in Eastern countries, and there have been numerous reports domestically. We analyzed 177 cases of hypertension caused by renal vascular lesions seen at Shanghai Zhongshan Hospital and Huashan Hospital from 1960 to 1984, of which 122 cases were caused by Takayasu arteritis. This finding differs significantly from reports in foreign literature. The primary lesion of Takayasu arteritis is in the aorta, characterized by panarteritis predominantly affecting the media. The media shows diffuse granulomatous tissue hyperplasia, accompanied by lymphocyte and plasma cell infiltration, with significant destruction or fragmentation of elastic fibers replaced by collagen. The vascular adventitia is thickened, with cell infiltration and tight adhesion to surrounding tissues. Intimal fibrosis, surface swelling, roughness, and thrombosis lead to stenosis of the renal artery orifice, impairing renal blood supply. The small intrarenal arteries generally show no hypertrophy or degenerative changes, and the intima exhibits no hyperplasia. These pathological changes suggest primary aortitis without atherosclerotic changes.
At Zhongshan Hospital, one case of bilateral renal artery occlusion causing hypertension died postoperatively from acute yellow liver atrophy. Autopsy revealed no lesions in the bilateral renal arteries, but significant inflammation in the aorta, with thickened arterial walls, patchy intimal hyperplasia, and narrowed lumens, particularly causing severe obstruction at the orifices of the bilateral renal arteries and the mesenteric artery. In 42 surgical cases, the abdominal aorta was explored, and 22 cases showed obvious thickening of the arterial wall, rough and irregular surfaces, hardened texture, and some with segmental dilation and stenosis, consistent with the characteristic manifestations of Takayasu arteritis. However, the resected kidneys, often cut distal to the stenosis, typically did not exhibit classic vascular lesions.
The exact cause of Takayasu arteritis is not yet fully understood, and it is currently considered an autoimmune disease. Ueda proposed that the disease can be clinically divided into three stages: ① acute active phase; ② chronic inflammatory phase; ③ scar stenosis phase. The clinical manifestations of the acute active phase are often not obvious and may include weakness, fever, night sweats, etc., which are easily overlooked, leading to delayed diagnosis. Subsequently, due to secondary allergic immune reactions, inflammation occurs in the aorta and its major branches, and when the renal artery orifice is involved, it can cause secondary hypertension. At the "National Seminar on Renovascular Hypertension" held in Shanghai in November 1988, 1,960 cases of renovascular hypertension were analyzed. According to the etiology, there were 1,087 cases of Takayasu arteritis, 279 cases of renal vascular malformation, 234 cases of atherosclerosis, 183 cases of fibromuscular hyperplasia, 43 cases of post-renal transplant hypertension, 18 cases of hypertension caused by external compression of the renal artery, 3 cases of traumatic thrombosis, 1 case of renal arteriovenous fistula, and 112 cases of unknown cause. Among these, Takayasu arteritis accounted for 55.4%, ranking first in incidence.
Regarding the diagnosis of renovascular hypertension, it is first necessary to exclude extrarenal diseases. Kidney excess parenchymal hypertension includes primary hypertension. Apart from a detailed medical history, the diagnosis of disease cause can generally be determined using routine examinations for urinary system diseases and certain specialized tests. For diagnosing renal vascular lesions, additional specialized examination methods are required.
(1) Medical History and Physical Examination Based on a review of the literature, the following factors should raise suspicion of hypertension caused by renal vascular lesions: ① Onset in youth, typically under 30 years of age (consistent with domestic data); ② Onset in the elderly, usually over 50 years of age; ③ Sudden exacerbation of long-term hypertension; ④ Sudden onset of hypertension with a short course or rapid progression; ⑤ Hypertension accompanied by back or flank pain; ⑥ Vascular murmurs audible in the abdomen or back; ⑦ No family history of hypertension; ⑧ Ineffectiveness or poor response to commonly used antihypertensive medications.
Recent literature has also presented differing views, suggesting that this condition can occur at any age and may not necessarily present with clinical symptoms. Even guiding medicinal effectiveness does not rule out the disease, and the presence of abdominal or back vascular murmurs is not absolutely diagnostic. Rivin found that 18% of 500 normal individuals had audible murmurs during abdominal auscultation. Maxwell noted that approximately half of renovascular hypertension cases exhibit vascular murmurs in the upper abdomen, with the incidence related to the nature of the lesion—higher in fibromuscular hyperplasia than in stirred pulse atherosclerosis. Domestic reports indicate that murmurs are audible in the upper abdomen in about two-thirds of cases (60–74%). We believe that abdominal vascular murmurs hold certain diagnostic significance for this condition.
(2) Excretory Urography (Intravenous Urography, IVU) Over the past 30 years, as understanding of renal vascular diseases and their hemodynamic effects has improved, the expectations for IVU have also increased. In terms of renal function, IVU should reflect changes in renal blood flow, glomerular filtration rate, and tubular reabsorption and secretion, serving as a basis for further screening, examination, and management of renal vascular diseases. In the 1960s and 1970s, many scholars recommended minute-sequence intravenous pyelography, which can reveal four major changes: ① Differences in kidney size; ② Differences in pyelogram appearance time; ③ Differences in contrast concentration in the renal pelvis; and ④ Ureteral notching. Other changes aiding the diagnosis of stirred pulse obstruction include reduced kidney width and calyceal length, kidney excess parenchymal atrophy, and shrinkage of the renal pelvis and calyces. Maxwell (1967) summarized over 500 cases of renal stirred pulse stenosis across eight studies, concluding that this method achieves a 90% positive rate for diagnosing renovascular hypertension. MacGregor (1975) noted a 15% false-positive rate and a 17–27% false-negative rate. Dean (1984), reviewing literature from the past decade, acknowledged the method’s modest positive rate but emphasized its ability to display renal morphology and function, exclude other renal diseases, and its simplicity and non-invasiveness, making it a viable initial screening tool.
(3) Split Renal Function Test In 1953, Howard introduced the bilateral ureteral catheterization method for split renal function testing to diagnose unilateral renal stirred pulse lesions, proving clinically useful. Subsequent modifications and additions by scholars have yet to perfect the method. Nonetheless, under limited conditions, the Howard and Stamey tests remain commonly used. The examination items and positive results (Table 1) are as follows:
Table 1 Howard and Stamey Tests
| Test Name | Test Item | Result (Affected Side) |
| Howard (1953) | Urine Volume (V) | Decreased by 50%↓ |
|
| Urinary Sodium (UNa) | Decreased by 15%↓ |
| Urinary Creatinine (Ucr) | Increased by 50%↑ | |
| Stamey (1961) | Urine Volume (V) | Decreased by 65%↓ |
|
| Urinary Creatinine (Ucr) | Increased by 100%↑ |
| Urinary Inulin (Uin) | Increased by 100%↑ |
Since these tests require ureteral catheterization to collect urine, they have drawbacks such as fistula disease, trauma, etc., making it difficult to control accuracy, and their positive criteria are also considered overly stringent. Deam (1984) reviewed 40 cases of unilateral renal artery main trunk occlusion with good postoperative outcomes, and only 20 cases met Howard's classic positive criteria. Therefore, the Vanderbilt Medical Center proposed using semi-classic criteria for detection (i.e., a 25% reduction in urine volume, a 25% increase in urinary creatinine, and a 50% increase in para-aminohippurate clearance CPAH), which could raise the positive rate to 72%. Dean also recommended collecting three urine samples for testing. If urine volume decreases while creatinine and PAH concentrations increase, it can also be classified as "positive," reducing the false-negative rate to 8%.
(4) Application of Radionuclides
1. Radionuclide Renogram This is a simple, safe, sensitive, and rapid method for measuring split renal function, aiding in the diagnosis of renovascular hypertension, and is now widely used. The a segment of the renogram reflects the radioactivity of the radionuclide reaching the renal vascular bed; the b segment is the secretory phase; and the c segment is the excretory phase. When renovascular hypertension affects renal function, the renogram may show abnormalities, manifesting as low or no function, with reduced vascular and secretory segments. If a rich collateral circulation has formed, the renogram may appear completely normal. Conversely, even if the renal artery is not obstructed, long-term sustained hypertension leading to renal arteriolar sclerosis may cause abnormal renogram results. The renogram only reflects changes in renal function and is therefore not specific; it cannot diagnose the cause of the disease. Segmental renal artery stenosis that has not yet affected renal function may not show abnormalities on the renogram.
2. Radionuclide Renal Scan The renal scan uses radionuclide-labeled compounds selectively concentrated and excreted by the kidneys, which are then imaged externally via a scanner. The resulting images are analyzed to compare the position, shape, size, and radioactive distribution density of both kidneys, combined with clinical conditions for diagnosis. When renal artery stenosis causes renal atrophy, the renal scan shows the affected kidney as smaller than normal, with sparser and uneven radioactive distribution. The contralateral kidney may exhibit compensatory hypertrophy. If renal artery stenosis has not yet caused functional changes, the renal scan may show no significant abnormalities.
3. Radionuclide Computed Tomography Chiarini (1982) proposed using 99mTc-DTPA as a tracer for dynamic gamma imaging of the bilateral renal regions to detect abnormalities in renal function and morphology. Under normal conditions, 0–2 seconds after the abdominal aortic pulse is visualized, the perfusion phase of both kidneys can be observed, with uniform and symmetrical radioactive distribution. During the parenchymal phase, the radioactivity in the renal regions peaks at 2–3 minutes. At 3–4 minutes, radioactivity begins to appear in the bladder area. Subsequently, the radioactivity in the renal regions gradually diminishes, while that in the bladder area increases, with the bladder region showing significantly higher radioactivity than the renal regions by 25 minutes. Chiarini used this technique to examine 30 cases of renovascular hypertension and found delayed perfusion phase and peak radioactivity in the affected kidney, with radioactivity distribution lower than that in the healthy kidney. The degree of reduction correlated with the severity of renal artery stenosis. The results indicated a positive rate of 89%, a false-positive rate of 10%, and a false-negative rate of 9%. Between 1985 and 1988, our hospital examined 16 cases of renovascular hypertension, with results similar to Chiarini's. In the initial stage [first stage], the images were black and white, but after enhancement processing and color synthesis, different grayscale levels were pseudo-colored, improving image resolution. Therefore, this examination is considered highly sensitive, simple to perform, free of adverse reactions, and particularly convenient for follow-up.
(5) Renin measurement, angiotensin blockers and converting enzyme inhibitor tests Among the diagnostic methods for renovascular hypertension, the measurement of renin, angiotensin blockers, and converting enzyme inhibitor tests have been highly regarded for many years.
1. Renin measurement
(1) Measurement of peripheral circulating renin activity The pressor effect of the renin-angiotensin system is well-established, but the relationship between renin levels and activity in the body and blood pressure is not simply parallel. This discrepancy is mainly related to the body's regulatory function over renin secretion. Due to the high rates of "false positives" and "false negatives" in peripheral circulating renin activity, diagnosis is challenging. However, recent studies suggest that if the peripheral circulating renin value is <5 nGAI/(ml·hr), renovascular hypertension can be largely ruled out; if it exceeds this value, it indicates a possible presence of renovascular hypertension, and further tests such as split renal vein renin activity or angiotensin blocker tests should be conducted.
(2) Split renal vein renin measurement: The ratio of renin activity between the two renal veins (affected side renin/contralateral side renin, RVRR), the level of peripheral circulating renin, or the ratio of contralateral renal vein renin to peripheral blood renin are measured. Currently, it is generally believed that when peripheral blood renin activity is high and the difference in renin activity between the two renal veins is greater than 2-fold, surgical outcomes are favorable. Kaufman reported an efficacy rate of 93%. When peripheral blood renin activity is normal or the ratio of contralateral renal vein to peripheral blood renin is below 1.3, and the difference in renin activity between the two renal veins is greater than 1.4-fold, postoperative blood pressure often returns to normal or significantly decreases. If the ratio of renin activity between the two sides is less than 1.4, surgical outcomes are poor, with a false-positive rate of about 7%.
Vaughan (1985) also suggested that when the renal vein renin ratio is 1.5:1, 90% of renovascular hypertension cases achieve good surgical outcomes. However, a negative ratio does not exclude satisfactory postoperative responses, with a false-negative rate of about 15% (62/412). On the other hand, a positive RVRR also cannot rule out bilateral sexually transmitted disease changes. Correcting one side of the lesion may not fully address the underlying pathological changes, so hypertension may still not be effectively controlled. Therefore, Vaughan proposed that the diagnostic criteria for renovascular hypertension should meet the following points: ① Use the renin-sodium index, i.e., demonstrate high renin secretion under stimulation by captopril; ② Confirm the absence of renin secretion on the contralateral side, V2-A2=0 (representing renal vein and renal stirred pulse renin values, respectively); ③ In unilateral high-renin renovascular hypertension, V-IVCV/IVC ≧ 0.5 (IVC refers to the inferior vena cava renin value), whereas primary hypertension differs, with both sides V-IVC/IVC equal to 0.25, regardless of whether peripheral renin is normal or elevated.
In recent years, many scholars have argued that the false-positive and false-negative rates of the renal vein renin ratio are relatively high, prompting calls for revisions and supplements. Dean et al. pointed out that left renal vein blood is not solely derived from the left renal vein but also from other branches, such as the adrenal vein, spermatic or ovarian vein, and lumbar vein. If the catheter is mistakenly inserted into other branches or placed in the proximal part of the renal vein, the collected blood sample may include reflux from other veins, diluting the plasma renin in the renal vein and creating artifacts. He also suggested that using a single catheter to collect blood from both renal veins for renin activity measurement often leads to errors. In cases with normal stirred pulse angiography, using a single catheter to collect blood from both renal veins for renin measurement resulted in a 24% rate of renal vein renin activity ratios exceeding 1.5 to 1.0. Among 39 surgically confirmed cases of unilateral renovascular hypertension, only 79% had a renal vein renin activity ratio exceeding 1.5. Whelton also reported a 22% false-positive rate (>1.5). Smith argued that using a single catheter to asynchronously collect blood samples from both renal veins for renin activity measurement is inaccurate. In 12 cases with normal stirred pulse angiography, Smith collected L1/R1, L2/R2, and L3/R3 ratios, which varied due to timing differences. Therefore, he advocated using two catheters to synchronously collect three sets of renal vein samples. If two of the renin activity values were similar, the results were deemed reliable, achieving a positive rate exceeding 90% in cases of unilateral renal stirred pulse stenosis hypertension.
2. Angiotensin Blockade Test (Angiotensin Blockade Test)
(1) Saralasin Test: This test involves replacing the aspartic acid at position 1 and phenylalanine at position 8 of angiotensin II with sarcosine and alanine, respectively. It competes with angiotensin II for receptors, causing a decrease in blood pressure without reducing endogenous angiotensin I. Positive indicators: ① A blood pressure drop of 4.0/2.6 kPa (30/20 mmHg) within 10 minutes; ② Diastolic pressure reduction ≥ 9.3%; ③ Plasma renin activity ≥ 14 ng AI/(ml·hr); ④ Renin activity response value/control value ≥ 2.2, indicating the patient has high-renin hypertension. 90–95% of patients with renovascular hypertension show positive results, and surgical outcomes are favorable. However, a small number of patients with high-renin essential hypertension may also exhibit a blood pressure-lowering response after saralasin injection, which should be noted.
(2) Saralasin-1, Threonine-8 AII Test: Novick (1983) suggested that the saralasin test often yields false positives and false negatives, proposing a new AII blocker called the saralasin-1, threonine-8 AII test. Compared to the saralasin test, it offers the following advantages: ① Minimal effect on aortic pulse contraction; ② Does not stimulate the adrenal medulla to increase catecholamine secretion; ③ Reduces peripheral vascular resistance; ④ Lowers blood pressure without decreasing cardiac output.
3. Converting Enzyme Inhibitor Test SQ20881 (Teprotide) is a converting enzyme inhibitor, a nonapeptide originally derived from snake venom and now synthetically produced. In animal experiments, after adrenalectomy and sodium restriction, the initial mean aortic pressure was maintained at 9.6 kPa (72 mmHg). Administration of SQ20881 immediately reduced blood pressure by 5.3 kPa (40 mmHg), while plasma renin activity increased from 6 ng AI/(ml·hr) to 120. Due to extracellular fluid restriction, blood pressure levels depend on renin and AII. The use of converting enzyme inhibitors leads to AII deficiency, causing blood pressure to drop to very low levels. The rise in renin is due to blood pressure reduction stimulating renal baroreceptors or inhibiting AII's negative feedback mechanism. Injecting AII and converting enzyme inhibitors can stabilize blood pressure, indicating that the inhibitor itself does not stimulate renin secretion. Positive results include: ① Diastolic pressure reduction ≥ 9.3%; ② Plasma renin activity ≥ 18 ng AI/(ml·hr); ③ Renin activity response value/control value ≥ 3.3.
(6) Abdominal Aortography Although some suggest that 3–32% of normotensive individuals may show varying degrees of renal artery stenosis in abdominal aortography, 67% of hypertensive patients exhibit renal artery stenosis. To date, abdominal aortography remains a critical diagnostic method for renovascular hypertension, providing definitive evidence and serving as the basis for surgical intervention.
In terms of angiography techniques, the percutaneous femoral artery catheterization method is now the most widely used due to its clear imaging, safety, and convenience.
1. In cases of renovascular hypertension, abdominal aortography primarily reveals the morphology of the abdominal aorta, renal arteries, their branches, and the parenchymal phase.
⑴Abdominal aortic imaging, especially near the renal artery orifice, for any abnormalities: In cases of abdominal aortic atherosclerosis and multiple abdominal aortitis, abnormal changes in the abdominal aorta can be observed, affecting one or both renal artery orifices, either as stenosis or occlusion. When the abdominal aortic stenosis is annular, it often leads to bilateral renal artery root stenosis.
(2) Understand the main trunk image of the renal artery and the presence of branches or aberrant vessels: When there is stenosis in the renal artery, observe the location, extent, degree of stenosis, and the presence of post-stenotic dilation. The nature of the lesion can be analyzed from the imaging morphology (Figures 1, 2, 3, 4): For atherosclerosis-induced lesions, the following may be observed: ① The renal artery appears irregularly patchy; ② Stenosis and obstruction often appear conical; ③ The stenosis is eccentric; ④ Calcification is present; ⑤ The lesion initially occurs at the opening or proximal segment of the renal artery; and ⑥ Other vessels in the abdomen may show signs of atherosclerosis. Changes due to fibromuscular dysplasia include: ① Long and smooth stenosis; ② Long and irregular beaded stenosis; ③ Scattered网状 stenosis; and ④ Lesions located in the middle and distal thirds of the renal artery. Changes due to polyarteritis nodosa include: ① The abdominal aortic lumen appears uneven or relatively uniform, with smooth-edged concentric stenosis or obstruction; ② A少数 cases show an expansive type, including aneurysm formation, some exhibiting both expansion and obstruction; ③ The renal artery shows localized stenosis and obstruction at the opening or proximal segment, accompanied by post-stenotic dilation; and ④ Polyarteritis nodosa is a multisystem disease that can affect the abdominal and descending aorta and its multiple branches.
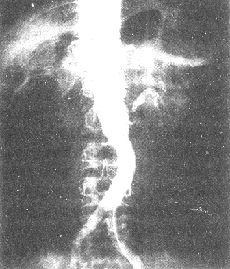
Figure 1 Abdominal aorta-renal artery angiography shows occlusion of the right renal artery and stenosis of the left renal artery.
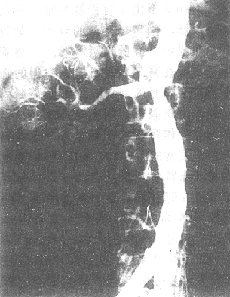
Figure 2 Abdominal aorta-renal artery angiography shows segmental stenosis-type aortitis complicated by renal artery stenosis.
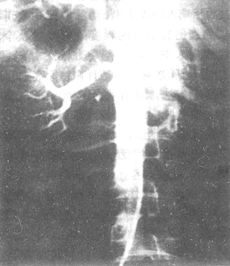
Figure 3 Abdominal aorta-renal artery angiography shows occlusive-type aortitis complicated by renal artery stenosis.
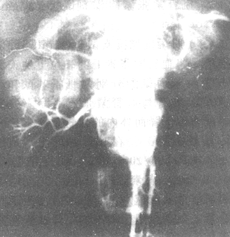
Figure 4 Abdominal aorta-renal artery angiography shows expansive-type aortitis complicated by renal artery stenosis.
(3) Post-stenotic dilation: Its incidence is related to the cause and degree of stenosis.
(4) Collateral circulation: When present, the arrangement is irregular, and the morphology is often tortuous. These vessels typically have a longer imaging time. The origins of collateral circulation are extensive, including the renal capsule, lumbar, intercostal, subphrenic, adrenal, gonadal, internal iliac, pelvic, and other arteries.
(5) Renal parenchymal imaging: During the renal parenchymal imaging phase, the renal parenchyma and轮廓 are clearly visualized, and the affected kidney is smaller than the contralateral healthy kidney. In cases of renal artery occlusion, the affected kidney may甚至 not be visualized at all.
According to data from our hospital's radiology department, 217 cases符合 polyarteritis nodosa were identified in angiography, of which 71 cases (32.7%) involved the renal artery and caused hypertension. The X-ray findings can sometimes be difficult to distinguish from atherosclerotic lesions. We propose the following points for differential diagnosis (Table 2).
Table 2 Differential diagnosis between atherosclerosis and polyarteritis nodosa in angiography
| Atherosclerosis | Polyarteritis nodosa |
| 1. Lesions are广泛, involving the thoracic and abdominal aorta | Lesions are widespread but scattered, sometimes localized |
| 2. The affected lumen is irregular, often enlarged,呈 aneurysm-like | The lumen at the lesion site is narrowed and grade I irregular, with rare stirred pulse tumors. |
| 3. The lumen at the lesion site is uneven and irregular | The lumen at the lesion site is generally smooth but may be accompanied by grade I irregularities |
| 4. The lesion site is twisted and elongated | The length of the lesion site mostly remains unchanged |
| 5. The lesion site shows uneven contrast density | The density at the lesion site is mostly uniform |
| 6. Filling defects are occasionally observed at the lesion site | No filling defects are present at the lesion site |
| 7. Calcification is present | Generally, no calcification shadows are observed |
Aortorenal artery angiography also has limitations in the diagnosis of renal artery stenosis, such as overlapping or unclear vascular images. Therefore, in some cases, selective or superselective angiography is required to accurately visualize the lesions in the various branches of the renal artery.
2. In terms of aortorenal artery angiography techniques, there have been many recent advancements, mainly including:
(1) Bookstein's pharmacological techniques can enhance the imaging of collateral circulation that is not clearly identifiable in conventional angiography. Injecting vasoconstrictors or vasodilators into the artery before renal angiography can make potential collateral circulation appear or disappear and alter blood flow direction. If conventional renal angiography does not reveal poststenotic nonparenchymal renal artery (PNPA) but it appears after epinephrine administration, or if PNPA is visible in conventional angiography but disappears after vasodilator acetylcholine, both phenomena indicate hemodynamic significance of the stenosis; otherwise, it is insignificant. The author believes this method is highly reliable for determining the hemodynamic significance of artery stenosis.
(2) Digital subtraction angiography (DSA): In 1981, Buonocore, Hillman, and others reported the application of DSA in renal angiography, highlighting its advantages of simplicity and clear imaging. DSA had been in use before the 1970s, involving the subtraction of pre-contrast images from direct arterial catheter angiography to eliminate non-vascular shadows (e.g., bones, soft tissues), resulting in clearer vascular images, known as subtraction angiography. In the late 1970s, the University of Wisconsin developed a digital video image processor, tested in animals and humans, and by 1980, DSA was successfully applied clinically. By the end of 1981, 700 cases were reported, with 71% involving the carotid artery, 12% the renal artery, 9% intracranial arteries, and 8% thoracic and peripheral vessels.
The resolution of DSA is sufficient to observe blood vessels with a diameter as small as 1mm within the kidney parenchyma. It can diagnose renal stirred pulse lesions with an accuracy of 91.1%, with 6.6% being of reference value, and only 2.3% of images failing to provide a diagnosis. DSA can differentiate conditions such as fibromuscular dysplasia, stirred pulse atherosclerosis, renal atrophy, renal stirred pulse narrowing, or renal stirred pulse occlusion. In cases where imaging is unsatisfactory, it may be due to extreme stenosis at the opening of the renal stirred pulse, resulting in insufficient contrast agent density affecting the visualization of small branches of renal blood vessels, or it could be caused by overlapping stirred pulse or insufficient cardiac output. DSA can measure values such as intrarenal blood distribution, perfusion status, accumulation function, and clearance function, thereby accurately assessing the physiological functions of both kidneys (Figures 5, 6).
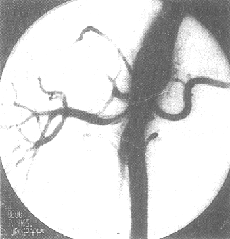
Figure 5 Abdominal aorta-renal stirred pulse angiography—DSA
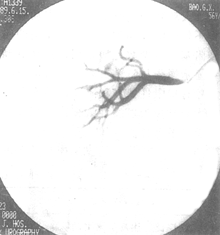
Figure 6 Renal stirred pulse angiography—DSA
(3) Application of pseudo-color enhancement image processing in renal stirred pulse angiography: "Pseudo-color enhancement image processing" is a new subject. The so-called "pseudo-color" is not the natural color of the original object, but rather the artificial coloring of black-and-white images. The human visual system can only distinguish 15–20 levels of grayscale, but can differentiate thousands of colors.
In recent years, pseudo-color images have been applied in aerospace, military, geological exploration, and oceanography, but their use in medicine is still a new endeavor. In 1984, Shanghai Zhongshan Hospital collaborated with the Precision Instrument Department of Shanghai Jiao Tong University to develop computer-based color synthesis technology for medical X-ray images, aiming to produce vivid, detailed, and realistic color X-ray images. Through this processing, the images can clearly display the abdominal aorta stirred pulse and its branches, as well as the main trunk and various branches of the renal stirred pulse. In cases of renal tumors, the internal density structure and edge morphology of the tumor can also be visualized. In renal angiography, to obtain clear vascular images and remove other artifacts from the angiograms, two X-ray images (pre- and post-contrast) are input into a computer. After alignment, subtraction processing is performed. This eliminates overlapping shadows of the spine, muscles, and intestines present in both images, leaving only the vascular image (i.e., DSA). The subtracted black-and-white image is then converted into a color image, resulting in a color vascular subtraction image.
Color synthesis technology involves transforming preprocessed black-and-white images into three separate images. Each image enhances a specific type of information, which is then fed into the red, green, and blue channels of a color display. The result is a color-synthesized image on the display. Different colors in the image represent information from different areas of the original picture. Density variations indistinguishable to the naked eye in the original image are displayed as distinct colors in the color image, making them easily discernible.
Practice has shown that medical X-ray image processing has broad prospects. In addition to X-ray images, it can also be applied to radionuclide imaging and CT images with similar processing effects.
bubble_chart Treatment Measures
The treatment of renovascular hypertension should adopt comprehensive measures and the integration of Chinese and Western medicine, which can be approached from three aspects: medical treatment, surgical treatment, and percutaneous transluminal angioplasty. Each therapy has its specific role and limitations. The management of any case depends on its specific condition and can involve combined applications. The goal of treatment is to restore adequate renal blood flow, control or reduce blood pressure, and improve renal function to alleviate symptoms and promote overall health recovery.
(1) Medical Treatment Medical treatment is used for cases unsuitable or unable to undergo surgery. Most patients experience some reduction in blood pressure through systemic therapy and medication. For those requiring surgery, medical treatment is also an important measure for preoperative preparation and postoperative management.
Medical treatment includes systemic care, dietary therapeutics, appropriate control of water and sodium intake, and medication. Antihypertensive drugs can be broadly categorized into the following types: ① natriuretic diuretics; ② sympathetic nerve inhibitors; ③ vasodilators; and ④ calcium antagonists. When selecting a reasonable drug treatment plan, it should be based on the pathophysiological foundation of the disease cause. Streeten et al., summarizing literature data, suggest that renovascular hypertension is mostly renin-dependent, and the use of angiotensin inhibitors can significantly lower blood pressure. Charer also points out that excessive renin production is the main cause of this condition, while increased blood volume or sodium retention is often associated with advanced-stage renal failure-related hypertension. It is also cited that the absence of certain vasodepressor substances (e.g., prostaglandins) and autonomic nervous system disorders are related to this condition. Zwifler further proposes that although all anti-adrenergic drugs can inhibit renin secretion, beta-blockers are the most effective, such as propranolol, clonidine, methyldopa, and guanethidine. If beta-blockers alone cannot control blood pressure, diuretics should be added. If severe damage to the contralateral kidney leads to increased blood volume as the main cause of hypertension, furosemide is the drug of choice. In recent years, angiotensin-converting enzyme inhibitors have gained increasing attention because they block the conversion of AI to AII. Following SQ20881, the oral drug SQ14225 (trade name captopril) has achieved good efficacy, but this drug may cause certain side effects due to the sulfhydryl group in its molecule, such as rash, neutropenia, and proteinuria. Since 1982, enalapril has been found to control blood pressure with a small dose of 10–20 mg daily, without the side effects of SQ14225.
(2) Surgical Treatment Since the 1950s, various renal artery reconstruction surgeries have been successively developed and achieved good results, making surgical treatment the general approach for renovascular hypertension. Except for a few cases, nephrectomy is now rarely performed, and various renal vascular reconstruction surgeries are selected based on specific lesions. This is because: ① This procedure preserves functional renal tissue, reduces the threat of contralateral renal compensatory dysfunction, and retains tissues that release antihypertensive substances; ② This procedure can salvage the so-called "healthy or better" contralateral kidney, whereas nephrectomy removes the affected kidney that still retains some function. The reason is that when unilateral renal artery stenosis causes hypertension, pressor factors affect the contralateral kidney of normal size, leading to certain irreversible vascular changes, while the affected kidney may be better protected due to its renal artery stenosis; ③ If this procedure is unsuccessful, a second surgery, including nephrectomy, can be performed, whereas nephrectomy is definitive and is only performed when the affected kidney is confirmed to be completely atrophic and nonfunctional; ④ This procedure can be used for cases of bilateral renal artery stenosis.
On the other hand, renal vascular reconstruction surgery can achieve good results for three reasons: ① A precise understanding of the anatomical and functional changes caused by renal stirred pulse lesions has led to clear surgical indications; ② Appropriate surgical methods have been selected, and operative techniques have improved, including extracorporeal intrarenal vascular reconstruction; ③ Surgical outcomes can be accurately predicted based on disease cause, renal vein renin ratio measurements, and split renal function tests.
The development of renal vascular reconstruction has a history of over 30 years. In 1954, Freeman first reported the successful treatment of a case of renal artery embolism using endarterectomy. In 1955, Hurwitt achieved success with a splenorenal artery anastomosis for a case of left renal artery obstruction. In 1956, Poutasse applied autologous artery transplantation to treat a case of bilateral renal artery stenosis. In 1960, Morris and DeBakey chose bypass surgery (by-pass operation) as a method for treating renovascular hypertension. Subsequently, other procedures such as autologous and artificial vessel grafts, artery patch augmentation, renal artery reimplantation, and renal autotransplantation have been reported. To date, there are many methods of renal vascular reconstruction, each with its own characteristics; the most suitable surgical method should be selected based on the specific condition.
Below is a brief description of several major renal vascular reconstruction techniques used in practice:
1. **Thromboendarterectomy** Applicable to atherosclerotic plaques or intimal hyperplasia at the renal artery orifice or the proximal one-third of the artery.
2. **Bypass Surgery (also called by-pass operation)** Suitable for cases of renal artery stenosis with post-stenotic dilation (Figure 7).
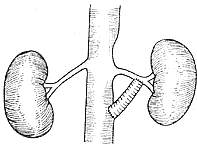
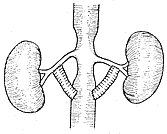
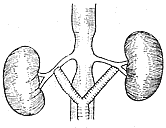
**Figure 7** Schematic diagram of abdominal aorta-renal artery bypass surgery
⑴ Unilateral bypass surgery ⑵ Bilateral bypass surgery ⑶ "Y"-shaped bilateral bypass surgery
3. **Splenorenal Artery Anastomosis** Suitable for left renal artery stenotic fibromuscular hyperplasia, provided the splenic artery is of sufficient size, as seen in preoperative aortography (recent reports suggest hepatorenal artery anastomosis for right renal artery stenosis with good results).
4. **Resection of the Stenotic Segment of the Renal Artery** Applicable to localized fibromuscular hyperplasia of the renal artery with a stenosis length of 1–2 cm.
5. **Resection and Graft Replacement** Suitable for renal artery stenosis exceeding 2 cm in length.
6. **Renal Artery Reimplantation** Suitable for abnormal renal artery origins or atherosclerotic plaques at the level of the renal artery orifice in the abdominal aorta. The distal end of the severed renal artery is reimplanted into a nearby normal segment of the abdominal aorta.
7. **Autologous Renal Transplantation (auto-renotransplantation, Figure 8)** The renal artery and vein are severed near the renal hilum, preserving longer segments of normal vessels. The kidney is cooled in 4°C saline, and 4°C renal perfusion solution is injected into the renal artery until the kidney turns uniformly pale and the venous outflow is clear. The kidney is typically transplanted into the ipsilateral iliac fossa, though some cases involve reimplantation into the original renal fossa.
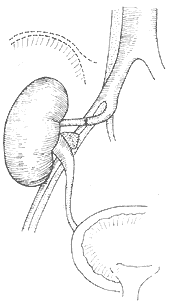
**Figure 8** Schematic diagram of autologous renal transplantation
The right kidney is transplanted into the right iliac fossa, with the right renal artery anastomosed end-to-end to the right internal iliac artery, the right renal vein anastomosed end-to-side to the right external iliac vein, and the ureter transplanted into the bladder.
Over the past two decades, numerous studies have reported the outcomes of surgical treatment for renovascular hypertension during the 1960s to 1980s. The general trend shows an increase in cure rates, improvement rates, and a significant reduction in mortality. A common point in these reports is that nephrectomies were performed primarily in the early stages. In 1971, Woods and Williams summarized the results of surgical treatments for renovascular hypertension across 12 groups from 1962 to 1967. Among 1,045 cases (248 nephrectomies and 797 vascular reconstructions), the average cure rate was 47%, improvement rate was 29%, and surgical mortality was 6%. In 1975, MacGregor compiled data from the literature on 2,281 surgically treated cases, reporting a cure rate of 50%, improvement rate of 30%, and mortality rate of 4%. In 1984, Stanley conducted a comprehensive analysis of 2,460 surgical cases from 11 centers between 1958 and 1980, with cure rates ranging from 37% to 66%, improvement rates from 19% to 54%, failure rates from 9% to 3.5%, and surgical mortality below 0.5%. Dean (1985) summarized the results of 706 surgeries performed between 1979 and 1984. Analysis by disease cause revealed that the cure rates for fibromuscular dysplasia and atherosclerotic lesions were 55–77% and 9–40%, respectively; improvement rates were 19–39% and 47–72%; and failure rates were 1–1.3% and 7–28%. Clearly, the fibromuscular dysplasia group outperformed the atherosclerotic group, with localized lesions in the latter showing better outcomes than diffuse lesions.
Generally speaking, the therapeutic effect in pediatric patients is higher than that in adults, with a cure rate of 58–85%, an improvement rate of 7–24%, and a failure rate of 0–7%.
In China, research papers on this subject began to be published in the 1960s. In the early stages, for hypertension caused by unilateral renal artery stenosis, nephrectomy or partial nephrectomy was often performed, with bypass surgery being the main method of vascular reconstruction. Although short-term results were satisfactory, long-term follow-up revealed that a significant number of cases developed restenosis at the anastomosis site, which is clearly related to the fact that the most common pathological basis of renovascular hypertension in China is multiple large arteritis. In 1976, Huashan Hospital in Shanghai reported the results of 22 cases treated with autologous renal transplantation. Among the 20 cases with long-term follow-up, 14 had normal blood pressure, 4 showed improvement, and 2 were ineffective. In 1984, an analysis of the treatment outcomes of 122 cases of renovascular hypertension was conducted, with 105 cases receiving long-term follow-up (ranging from 1 to 22 years, half of which exceeded 5 years). Among the 30 cases treated medically, only 10 survived, with blood pressure remaining high. Of the 18 cases treated with nephrectomy or partial nephrectomy, blood pressure initially returned to normal but later rose again. Among the 25 cases treated with vascular reconstruction (mostly bypass surgery), most showed good short-term results, though a few experienced a recurrence of high blood pressure, with two cases (3 and 16 years post-surgery) confirmed by repeat angiography to have restenosis at the bypass anastomosis site. Possible contributing factors include: progression of aortic arteritis lesions, foreign body reactions at the anastomosis site due to artificial grafts, or atrophy of autologous veins. Splenorenal artery anastomosis yielded poor results, likely due to the low perfusion pressure of the splenic artery. Among the 48 cases of autologous renal transplantation (including the 22 cases from Huashan Hospital), 4 with accompanying aortic aneurysms died (1 postoperatively, and 3 at 5, 13, and 19 months post-surgery due to other causes). Blood pressure remained normal in 28 cases, significantly improved in 12, and was unsatisfactory in 4, resulting in an overall effectiveness rate of 83.3%. Currently, autologous renal transplantation has been widely adopted in China, with incomplete statistics reporting 212 cases across two groups, showing short-term effectiveness rates of 82% and 95.2%, and long-term effectiveness of 79.8%. We believe that, in China, autologous renal transplantation is generally the preferred surgical method for treating renovascular hypertension due to the following advantages: ① It avoids the use of artificial or autologous vessels, thus preventing foreign body reactions or atrophy at the anastomosis site; ② Large arteritis typically does not affect the internal iliac artery, ensuring adequate blood supply to the transplanted kidney; ③ The surgical field is well-exposed and easy to operate on; ④ It is also suitable for bilateral lesions. However, if lower limb blood pressure is lower than upper limb blood pressure, bypass surgery should first be performed at the proximal and distal ends of the aortic stenosis or aneurysm before autologous renal transplantation, otherwise the results may be unsatisfactory.
Over the past decade, for cases where in situ renal vascular reconstruction is not feasible, ex vivo reconstruction (bench surgery) has opened a new pathway. The surgical procedure consists of three steps: ① Freeing the renal hilum vessels, temporarily exteriorizing (removing) the kidney, and protecting it with hypothermic perfusion; ② Using microsurgical techniques to separate the relevant branches of the renal artery, addressing the lesions, and performing vascular repair and reconstruction; and ③ Renal re-transplantation (either in the original renal fossa or the iliac fossa). In 1984, Stoney reported the results of 24 cases of ex vivo vascular reconstruction, with postoperative angiography confirming success in 22 cases; the remaining case awaited treatment for contralateral renal artery lesions. In 1988, Najarian reported the outcomes of 39 such surgeries, with 36 successes and 3 failures.
In the performance of bypass and replacement surgeries, recent literature has discussed more on the selection of grafts. Most authors prefer autologous veins due to their convenience in harvesting, achieving good results in 85–90% of cases; some have applied the internal iliac stirred pulse, with an efficacy rate of up to 98%. Over the past decade, the exploration of artificial blood vessels as grafts has drawn attention. Most use Dacron, while others recommend porous expanded polytetrafluoroethylene (PTFE). Synthetic grafts offer flexible sourcing, but thrombus formation imposes certain limitations on their applicability. Based on this, research on endothelial cell seeding in artificial blood vessels has gained increasing attention.
In 1978, Herring first successfully used a mechanical method to seed venous endothelial cells onto the inner surface of artificial blood vessels. Shortly thereafter, the University of Michigan replaced this technique with an enzymatic approach, where harvested venous endothelial cells were cultured in a solution of collagenase and trypsin before being seeded onto preclotted artificial vascular grafts. Experiments demonstrated that at least 80% of the lumen area was covered by the seeded endothelial cells after 4 weeks, and complete coverage could be achieved by 4 months without thrombus formation. Other researchers have reported similar findings. Endothelialization of artificial blood vessels also enhances resistance to bacterial infections, as the endothelial cell layer prevents bacterial adhesion. Building on animal experiments, Indiana University published two clinical reports in 1984 and 1987 on the application of endothelial cell seeding in artificial blood vessels. The results showed that after one and a half years, the patency rates of seeded versus unseeded grafts were 100% and 60%, respectively. Additionally, smokers exhibited significantly lower patency rates compared to non-smokers.
The mechanism of endothelialization in artificial blood vessels has a theoretical basis. The membranes of animal vascular endothelial cells contain arachidonic acid phospholipids, which release arachidonic acid under the action of phospholipase. The latter, under the influence of cyclooxygenase and prostacyclin synthase, synthesizes prostacyclin (PGI2), which inhibits platelet aggregation, disperses platelet aggregates, and exerts strong vasodilatory effects. This action antagonizes that of thromboxane A2 (TXA2).
(3) Percutaneous transluminal angioplasty (PTA) In 1978, Grüntzig first successfully used PTA to dilate renal artery stenosis, opening a new approach for the treatment of renovascular hypertension. Since then, PTA has been rapidly adopted in clinical practice. By 1980, Dotter estimated that over 15,000 PTA procedures had been performed in Europe and the United States. PTA operates on the principle of coaxial vascular dilation. A catheter with a balloon is inserted through the renal artery stenosis, and the balloon is inflated to an appropriate pressure (approximately 5 atmospheres) to increase the lumen diameter. Technical success can be confirmed by post-procedure angiography, with a general success rate of 97%. Numerous reports indicate that 90% or more of patients with renovascular hypertension experience significant blood pressure reduction within one month. Tegtmeyer followed 80 cases of atherosclerotic involvement for 1–52 months, with 25 cases cured, 47 improved, and 8 showing no effect. Martin summarized the results of 111 PTA cases across five groups: technical success was achiev






