| disease | Pain Wind and Hyperuricemia |
| alias | Gout, Hyperuricemia |
Gout is a group of diseases caused by purine metabolism disorders, characterized clinically by hyperuricemia and the resulting recurrent acute gouty arthritis, gout stone deposition, chronic gouty arthritis, and joint deformities. It often affects the kidneys, leading to chronic interstitial nephritis and the formation of uric acid kidney stones. This condition can be divided into primary and secondary categories. The cause of primary gout remains largely unclear, except for a few cases caused by enzyme defects, and is often associated with hyperlipidemia, obesity, diabetes, hypertension, arteriosclerosis, and coronary heart disease, being a hereditary disease. Secondary gout can arise from various causes such as kidney disease, blood disorders, and medications.
bubble_chart Etiology
(1) Hyperuricemia Uric acid in the human body has two sources: the exogenous source comes from the breakdown of nucleotides in foods rich in nucleoproteins, while the endogenous source arises from the synthesis of amino acids, phosphoribose, and other small molecular compounds, as well as nucleic acid catabolism. In the development of hyperuricemia, endogenous metabolic disorders are more significant than exogenous factors. Isotope tracer studies show that the average uric acid pool in a normal human body is 1,200 mg, with approximately 750 mg produced daily and 500–1,000 mg excreted. About two-thirds is excreted through urine, while the remaining one-third is eliminated via the intestines or broken down by intestinal bacteria. In a normal human body, over 99% of uric acid in the bloodstream exists in the form of sodium urate (referred to as urate). Serum urate levels fluctuate within a narrow range, with domestic data indicating an average of 5.7 mg/dl for males and 4.3 mg/dl for females.
Hyperuricemia is a key marker of gout. Elevated blood urate concentrations can result from either increased uric acid production or decreased uric acid excretion. Uric acid is the final product of human purine metabolism, and urate concentrations are closely related to purine metabolism. The feedback regulation of purine metabolism and the pathways of uric acid synthesis are illustrated in Figure 1.
Figure 1: Feedback Regulation of Purine Metabolism and Pathways of Uric Acid Synthesis
The first step in purine metabolism and its feedback inhibition site involves the reaction of phosphoribosyl pyrophosphate (PRPP) + glutamine → phosphoribosylamine + glutamate, catalyzed by phosphoribosyl pyrophosphate amidotransferase (PRPPAT). Several mechanisms can increase purine synthesis: ① Elevated substrate levels of PRPP or/and glutamine; ② Increased enzyme quantity or activity, or reduced sensitivity to feedback inhibition by purine nucleotides; ③ Decreased levels of adenosine monophosphate (AMP) or guanosine monophosphate (GMP), leading to reduced enzyme inhibition. These factors can accelerate purine synthesis and increase uric acid production. In some hyperuricemia patients, the condition is caused by a deficiency of hypoxanthine-guanine phosphoribosyltransferase (HGPRT). This enzyme facilitates the conversion of hypoxanthine to inosine monophosphate (IMP) and guanine to guanosine monophosphate (GMP). When HGPRT is deficient, PRPP consumption decreases, leading to an accumulation of PRPP and accelerated purine synthesis, thereby increasing uric acid production.
A small proportion of primary gout patients do not exhibit increased uric acid production; instead, their hyperuricemia is primarily due to reduced renal clearance. The excretion of urate by the kidneys is a complex process. Urate freely passes through the glomerulus, but almost all filtered urate is reabsorbed by the proximal tubules (pre-secretory reabsorption). Subsequently, the renal tubules secrete urate, and a portion of the secreted urate is reabsorbed again (post-secretory reabsorption). Reduced glomerular filtration, increased tubular reabsorption of urate, or decreased tubular secretion of urate can all lead to reduced urate excretion, resulting in hyperuricemia.
(II) Acute attack of gouty arthritis is an acute inflammatory reaction caused by the deposition of sodium urate (referred to as urate) in the form of crystals in the joints and surrounding tissues. The solubility of urate under normal physiological conditions, i.e., pH 7.4 and temperature 37°C, is 6.4 mg/dl. When the concentration of urate in body fluids increases to a supersaturated state, under certain triggering conditions such as injury, local temperature reduction, local pH decrease, or systemic fatigue and alcohol abuse, it is prone to crystallize. Urate crystals can chemotactically attract white blood cells. After white blood cells and the inner layer cells of the synovial membrane in the joint capsule phagocytose urate, they can release leukotriene B4 (LTB4) and glycoprotein chemotactic factors within minutes. In vitro experiments also show that monocytes can be stimulated by urate crystals and release interleukin-1 (IL-1), which can trigger and exacerbate gouty arthritis inflammation. The production of these factors can be inhibited by colchicine, hence colchicine can effectively stop the onset of gouty arthritis. After urate crystals are phagocytosed by cells, they quickly damage the phagolysosome membrane, releasing hydrolytic enzymes, causing white blood cell necrosis and releasing various inflammatory factors such as kinins, leading to acute inflammation. The phospholipid membranes of organelles, if containing cholesterol and testosterone, are sensitive to the cytolytic reaction induced by urate, whereas if containing β-estradiol, they are resistant. This explains why gouty arthritis is more common in males and postmenopausal women. The joints of the lower limbs, especially the big toe, bear the most pressure, are prone to injury, and have lower local temperatures, making them the most common sites for gouty arthritis.
(四)Renal lesions in gout Gout patients often exhibit renal damage, primarily manifesting in three forms:
1. Gouty nephropathy The characteristic histological feature of gouty kidney is the presence of urate crystals in the renal medulla or papillae, surrounded by round cells and giant cell reactions. Autopsies of gout patients frequently reveal these gouty kidney manifestations, often accompanied by acute and chronic interstitial inflammatory changes, fibrosis, tubular atrophy, glomerulosclerosis, and renal arteriolosclerosis. Gouty nephropathy is generally considered a grade I slow-progressing lesion, but it often becomes complicated by factors such as hypertensive arteriolosclerosis, urinary tract stones, and infections, making the renal changes in gout highly complex in terms of onset, progression, pathology, and prognosis.
2. Acute obstructive nephropathy Due to the deposition of uric acid (not urate) crystals in the renal collecting ducts, renal pelvis, calyces, and ureters, urinary flow obstruction leads to acute renal failure. This is commonly seen in patients with grade III hyperuricemia, such as those with myeloproliferative disorders undergoing chemotherapy or radiotherapy, where massive urate production occurs.
3. Uric acid nephrolithiasis The incidence of kidney stones in gout patients is 200 times higher than in the general population, approximately 35–40%. Among these, 84% are pure uric acid (not urate) stones, 4% are mixed uric acid and calcium oxalate stones, and the remainder are oxalate or calcium phosphate stones. The incidence of stones increases with higher serum urate concentrations and greater urinary uric acid excretion. When serum urate exceeds 12 mg/dL or urinary uric acid excretion exceeds 1100 mg/day, half of the patients develop kidney stones. The pKa of uric acid is 5.75; at plasma pH 7.4, over 99% exists in ionized form (sodium urate), whereas at urine pH 5.0, 85% is non-ionized (uric acid). Only 15 mg of uric acid dissolves per 100 mL of urine, and persistent acidic urine facilitates uric acid stone formation. Alkalinizing urine to pH 7.0 increases uric acid solubility tenfold.
(五)The disease can be classified into primary and secondary types, as shown in Table 1:
Table 1 Classification of gout and hyperuricemia
| Type | Uric acid metabolism disorder | Genetic characteristics |
| Primary | ||
| (1) Idiopathic | ||
| 1. Urinary uric acid excretion | Overproduction and/or reduced renal clearance | Polygenic |
| 2.Increased urinary uric acid excretion | Excessive Unprocessed Rehmannia Root: with or without reduced renal clearance | Polygenic |
| (2) Associated with enzyme and metabolic defects | ||
| 1.Increased PRPP synthetase activity | Overproduction; increased PRPP synthesis | X-linked |
| 2.Partial HGPRT deficiency | Overproduction; increased PRPP concentration | X-linked |
| Secondary | ||
| (1) Associated with increased purine synthesis | ||
| 1.Complete HGPRT deficiency | Overproduction; Lesch Nyhan syndrome | X-linked |
| 2.Glucose-6-phosphatase deficiency | Overproduction and reduced renal clearance; glycogen storage disease type Ⅰ (Von Gierke disease) | Autosomal recessive |
| (2) Associated with increased nucleic acid turnover | Overproduction; such as chronic hemolytic anemia, polycythemia, myeloproliferative disorders, and during chemotherapy or radiotherapy | |
| (3) Associated with reduced renal uric acid clearance | Renal dysfunction; due to drugs, toxins, or endogenous metabolites inhibiting uric acid excretion and/or increased reabsorption |
bubble_chart Clinical Manifestations
Primary pain wind was previously considered relatively rare in our country, but in recent years, due to improved nutritional conditions, increased average lifespan, and greater attention to the disease, it has been discovered more frequently. The prevalence gradually increases with age and is more common in men, with a male-to-female ratio of approximately 20:1. Women rarely develop the disease, and if it occurs, it mostly happens after menopause. Many cases reported abroad have a positive family history, mostly autosomal dominant inheritance, with a few being sex-linked inheritance. It is more prevalent among mental workers and those in economically and nutritionally advantaged social strata.
The natural course and clinical manifestations of pain wind can generally be divided into the following four stages: ① Asymptomatic hyperuricemia, ② Acute gouty arthritis stage of attack, ③ Inter-critical gout (pain wind remission period), ④ Chronic tophaceous gouty arthritis.
(1) Asymptomatic hyperuricemia: Serum urate concentration increases with age and varies by gender. There is no difference between boys and girls in childhood, with an average level of 3.6 mg%. After puberty, men have about 1 mg% higher levels than women, and the difference narrows again after menopause. Therefore, hyperuricemia can occur in men after adolescence, while in women, it usually develops after menopause. Many individuals with hyperuricemia may remain asymptomatic throughout their lives, referred to as asymptomatic hyperuricemia. Only when arthritis occurs is it called pain wind. The higher and longer the serum urate concentration remains elevated, the greater the likelihood of developing pain wind and urinary tract stones. The peak age of onset for pain wind is around 40 years old.
(2) Acute gouty arthritis: This is the most common initial symptom of primary pain wind, predominantly affecting the lower limb joints. A typical attack begins suddenly—patients may go to bed healthy but wake up in the middle of the night due to severe foot pain. Symptoms peak within hours, with marked redness, swelling, heat, and intense pain in the joints and surrounding soft tissues, sometimes so severe that even the weight of a blanket is unbearable. Large joint involvement may lead to effusion. Systemic symptoms such as headache, fever, and leukocytosis may also accompany the attack. Most patients experience no prodromal symptoms, but some report fatigue, general discomfort, or localized joint stabbing pain before the onset. Over half of the patients experience their first attack in the  toe, and about 90% of patients eventually have the big
toe, and about 90% of patients eventually have the big  toe affected during the course of the disease. The metatarsophalangeal joints, ankles, knees, fingers, wrists, and elbows are also common sites, while the shoulders, hips, and spine are less frequently involved. The first attack usually affects a single joint, but repeated episodes may involve multiple joints. Attacks can occur year-round but are more common in spring and autumn. Onset at midnight is typical. Local joint injury, such as foot sprain, wearing tight shoes and walking excessively, surgical procedures, heavy meals with alcohol, excessive fatigue, exposure to cold and dampness, and infections may serve as triggering factors.
toe affected during the course of the disease. The metatarsophalangeal joints, ankles, knees, fingers, wrists, and elbows are also common sites, while the shoulders, hips, and spine are less frequently involved. The first attack usually affects a single joint, but repeated episodes may involve multiple joints. Attacks can occur year-round but are more common in spring and autumn. Onset at midnight is typical. Local joint injury, such as foot sprain, wearing tight shoes and walking excessively, surgical procedures, heavy meals with alcohol, excessive fatigue, exposure to cold and dampness, and infections may serve as triggering factors.
pain wind The attack lasts for several days to several weeks and can naturally alleviate, with joint mobility fully restored, leaving only traces such as changes in skin color in the inflamed area. This is followed by an asymptomatic phase, known as the intermission period, lasting months, years, or even over a decade. Most patients experience a relapse within a year, after which episodes occur several times annually or once every few years. In rare cases, a patient may have only one episode in their lifetime. A significant proportion of patients tend to have increasingly frequent attacks, with more joints becoming affected, leading to chronic arthritis and joint deformities. Only a very small number of patients progress directly from the initial attack to chronic arthritis without any intermission period.
(3) Gouty tophi and chronic arthritis In untreated patients, the deposition of urate crystals in the joints increases, and recurrent inflammatory episodes progress to a chronic stage without complete resolution. This leads to bone erosion, defects in the joints, and fibrosis of surrounding tissues, resulting in joint stiffness, deformity, and restricted movement. On the basis of chronic changes, acute inflammation may still recur repeatedly, worsening the condition and making the deformities more pronounced, severely affecting joint function. In some patients, acute symptoms are mild or atypical, and the condition is only discovered after joint deformities appear. A few cases of chronic arthritis may affect all joints, including large joints such as the shoulders and hips, as well as the spine. Additionally, urate crystals may deposit in tendons, tendon sheaths, and connective tissues near the joints, forming yellowish-white, variably sized protrusions known as **gouty tophi** (or **gout stones**). These can range in size from as small as a **sesame seed** to as large as an **egg** or bigger. They commonly occur on the helix of the ear, extensor surfaces of the forearms, **metatarsal** toes, fingers, elbows, etc., but do not affect the liver, spleen, lungs, or central nervous system (Figures 2, 3). Initially, the nodules are soft, but as fibrous tissue proliferates, they become increasingly hard. In areas prone to friction, the overlying skin becomes thin and may ulcerate, forming fistulas that discharge white, powdery urate crystals. However, due to the bacteriostatic effect of urate, secondary infections are rare. The surrounding tissue of the fistula exhibits chronic inflammatory granulomas and is difficult to heal. The occurrence of **gouty tophi** is related to the duration of the disease and the level of **hyperuricemia**. Generally, literature reports that among patients with serum uric acid levels below 8 mg/dL, 90% do not develop **gouty tophi**, whereas in those with levels exceeding 9 mg/dL, 50% develop them. The longer the disease duration, the higher the likelihood of **gouty tophi** formation. Soft nodules that have formed recently may gradually shrink or even disappear with a low-purine diet and urate-lowering medications. However, long-standing, hard nodules are less likely to resolve due to fibrous proliferation.
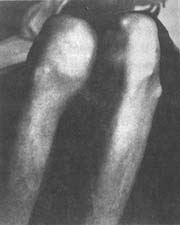
Figure 2 Gout
Left knee joint with **gouty tophi**
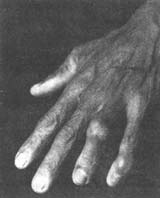
Figure 3 Gout
Deformity of the left ring and little fingers with **gouty tophi** formation
(4) Renal manifestations About one-third of long-standing **gout** patients exhibit kidney damage, which manifests in three forms:
1. **Gouty nephropathy** – Urate crystal deposition in renal tissue causes interstitial nephritis. Early stages may present only with proteinuria and microscopic **hematuria**, which can be intermittent and easily overlooked. As the disease progresses, proteinuria becomes persistent, and renal function—particularly concentrating ability—is impaired, leading to symptoms such as nocturia and low urine specific gravity. Further progression results in chronic azotemia and eventually uremia. Historically, about 17–25% of **gout** patients died from renal failure. Since **gout** patients often have comorbidities such as hypertension, **arteriosclerosis**, kidney stones, and urinary tract infections, **gouty nephropathy** may result from multiple factors.
2. **Acute renal failure** – Extensive blockage of renal tubules by large amounts of urate crystals can lead to urinary obstruction and acute renal failure. Prompt treatment, such as increased fluid intake, alkaline medications, and uric acid reduction, can often reverse the condition.
3. **Urinary tract stones** – About 20–25% of primary **gout** patients develop uric acid urinary stones. In some cases, kidney stone symptoms precede arthritis onset. Secondary hyperuricemia increases the risk of urinary stones. Small, sand-like stones may pass asymptomatically, while larger ones often cause **renal colic**, **hematuria**, and urinary tract infections. Pure uric acid stones are radiolucent and invisible on X-rays, but those mixed with calcium salts may be detected on plain radiographs.
Pain wind patients often have hypertension, hyperlipidemia, stirred pulse sclerosis, coronary heart disease, and diabetes (type II). Among the causes of death in elderly patients, heart blood vessel factors far exceed those of renal insufficiency. Regarding the connection between pain wind and the aforementioned diseases, it is generally believed that there is no direct causal relationship, but rather it may be related to common factors such as obesity, diet, and alcohol consumption. Restricting diet and reducing body weight can often help control hyperuricemia, diabetes, hypertension, and hyperlipidemia.
Secondary pain wind mostly occurs in myeloproliferative diseases such as acute and chronic leukemia, polycythemia, multiple myeloma, hemolytic anemia, lymphoma, and during chemotherapy for various cancers, where massive nucleic acid decomposition in cells leads to excessive uric acid production; or in renal diseases, hypertension, advanced-stage stirred pulse sclerosis, where renal failure makes uric acid excretion difficult, resulting in elevated hematuria acid. The hematuria acid concentration in secondary pain wind patients is often higher than in primary cases, and the incidence of urinary stones is also higher. However, due to the typically shorter disease course, joint symptoms are less typical than in primary cases and are often masked by the primary disease, making them difficult to detect. Since most patients are critically ill and have a short lifespan, various chronic-phase manifestations are relatively rare. Additionally, drug-induced hyperuricemia often occurs with the use of thiazide diuretics, ethacrynic acid, furosemide, and vinegar nitrogen amide. Sodium salicylate has a uricosuric effect at high doses but inhibits renal tubular excretion of uric acid at low doses, leading to elevated hematuria acid. Chronic lead poisoning can cause hyperuricemia and pain wind due to kidney damage.
Pain wind in adolescents and children is a rare condition, occasionally seen in type I glycogen storage disease. Due to glucose-6-phosphatase deficiency, blood sugar decreases, promoting increased glycogen breakdown and excessive lactic acid production, which inhibits renal tubular excretion of uric acid. Meanwhile, nucleotide consumption increases purine synthesis, resulting in hyperuricemia. Patients primarily present with episodic hypoglycemia. Another condition is Lesch-Nyhan syndrome, caused by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) deficiency, leading to increased uric acid synthesis and marked hyperuricemia. This syndrome occurs in male infants under one year of age, often presenting with cerebral paralysis, intellectual impairment, choreoathetosis, and primary pain wind symptoms. Milder cases may not manifest until adolescence, with no disabling signs, and pain wind symptoms may be the first noticeable feature. Patients excrete large amounts of uric acid in urine, and uric acid stones are often the initial symptom. Neurological symptoms appear in only 20% of cases, sometimes presenting solely as grade I spinal-cerebellar ataxia.
bubble_chart Auxiliary Examination
(1) Serum urate measurement Different detection methods yield varying results. The normal value for men using the uricase method is generally 7 mg/dl abroad, while women are about 1 mg/dl lower than men. Pain wind patients are often accompanied by elevated hematuria urate. However, due to the inherent variability of uric acid (e.g., increased adrenal cortex hormone secretion during acute episodes, enhancing uric acid excretion), as well as factors like water intake, diuresis, and medications, hematuria urate levels may sometimes appear normal. Repeated testing is necessary to avoid misdiagnosis of fistula disease.
(2) Urine uric acid measurement This is not very helpful in diagnosing acute arthritis, as more than half of pain wind patients have normal urinary uric acid excretion. However, urine tests can provide insights into uric acid excretion, aiding in drug selection and differentiating whether urinary stones are caused by elevated uric acid. Under normal dietary conditions, 24-hour urinary uric acid excretion is below 600 mg.
(3) Synovial fluid examination During acute episodes, if larger joints such as the ankle or knee are swollen, synovial fluid can be extracted for polarized light microscopy. Needle-shaped sodium urate crystals with birefringence can be observed within white blood cells, which is diagnostically significant. The positive rate under optical microscopy is only half that of polarized light microscopy. Synovial fluid analysis is also helpful, with white blood cell counts typically ranging from 1,000 to 7,000 and potentially reaching 50,000, predominantly segmented neutrophils.
(4) X-ray examination In early acute arthritis, apart from soft tissue swelling, joint imaging appears normal. Bone changes only occur after repeated episodes, starting with the destruction of the articular cartilage margin, irregular joint surfaces, and narrowing of the joint space. As the disease progresses, pain wind stone deposits can be seen in the subchondral bone and bone marrow. The bone exhibits punched-out defects, with sharp edges regardless of size, appearing semicircular or in continuous arcs. There may also be proliferative reactions at the bone margins (Figure 4).
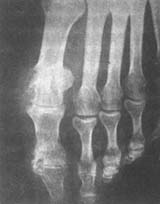
Figure 4 Pain wind
The left metatarsophalangeal and interphalangeal joints show punched-out defects with osteophytes.
(5) Special examinations for pain wind stones Biopsy can be performed on pain wind nodules, or special chemical tests (e.g., Murexide) can be used for identification. Additionally, ultraviolet spectrophotometry and uricase decomposition assays can be conducted.
Middle-aged or older men who suddenly experience redness, swelling, and pain in a single joint such as the big toe, metatarsus, ankle, or knee, accompanied by elevated urate levels and hematuria, should be suspected of having gout. The diagnosis can be confirmed by finding urate crystals in synovial fluid. Generally, the diagnosis is not difficult.
bubble_chart Treatment Measures
The prevention and treatment of this disease, whether primary or secondary, mostly lack disease cause therapy except for a few cases caused by drugs that can be discontinued, and therefore cannot be cured. Clinical treatment aims to achieve the following four objectives: ① Terminate acute arthritis attacks as soon as possible; ② Prevent recurrence of arthritis; ③ Correct hyperuricemia and prevent complications caused by urate deposition in the kidneys, joints, etc.; ④ Prevent the formation of uric acid kidney stones.
(1) Treatment of the acute stage of attack: Patients should rest in bed and elevate the affected limb, generally until 72 hours after arthralgia relief before resuming activity. Drug treatment should be initiated as early as possible, as early treatment can rapidly alleviate symptoms, whereas delayed treatment makes inflammation harder to control. Commonly used drugs include the following:
1. Colchicine: Highly effective for this disease. Initially, 0.5mg hourly or 1mg every 2 hours until symptoms are relieved or gastrointestinal side effects such as nausea, vomiting, and diarrhea occur. Generally, 4–8mg is required, with symptoms improving within 6–12 hours and controlled within 24–48 hours. Subsequently, 0.5mg can be given two to three times daily for several days before discontinuation. For severe gastrointestinal reactions, 1–2mg of the drug can be dissolved in 200ml of saline and slowly injected intravenously over 5–10 minutes, but care must be taken to avoid guiding medicinal extravasation. Another injection can be given after 6–8 hours if needed. For patients with renal impairment, the dose should not exceed 3mg within 24 hours. Due to its remarkable clinical efficacy, it can be used as a diagnostic trial for difficult cases to aid differential diagnosis. During colchicine treatment, attention should be paid to reactions such as leukopenia and hair loss.
2. Phenylbutazone or oxyphenbutazone: These have significant anti-inflammatory effects and promote uric acid excretion, remaining effective even several days after onset. The initial dose is 0.2–0.4g, followed by 0.1g every 4–6 hours. After symptom improvement, reduce to 0.1g three times daily for several days before discontinuation. These drugs can cause gastritis and water-sodium retention and are contraindicated in patients with active ulcers and cardiac insufficiency. Side effects such as leukopenia and thrombocytopenia may occasionally occur.
3. Indomethacin: Initial dose of 25–50mg every 8 hours, reduced to 25mg two to three times daily for 2–3 days after symptom relief. Its efficacy is similar to phenylbutazone, with side effects including gastrointestinal irritation, water-sodium retention, dizziness, headache, and rash. It is contraindicated in patients with active peptic ulcers.
4. Ibuprofen: A non-steroidal anti-inflammatory and analgesic drug. 0.2–0.4g two to three times daily can rapidly control acute symptoms within 2–3 days. This drug has fewer side effects, with no significant impact on blood counts or renal function. Occasional gastrointestinal reactions and elevated transaminases may occur.
5. Piroxicam: Long-lasting effect, administered once daily at 20mg. Occasional gastrointestinal reactions may occur. Long-term use requires monitoring of blood counts and liver/kidney function.
6. Naproxen: A non-steroidal anti-inflammatory and analgesic drug with anti-inflammatory effects 11 times that of phenylbutazone and analgesic effects 7 times that of aspirin. It has fewer gastrointestinal side effects. Oral dose is 500–750mg daily, divided into two doses.
7. ACTH and prednisone: For severe cases unresponsive to colchicine, ACTH 25mg can be added to glucose for intravenous drip, or 40–80mg can be injected intramuscularly. These drugs act rapidly but may cause rebound recurrence after discontinuation. Adding colchicine 0.5mg two to three times daily can prevent rebound. Triamcinolone hexacetonide 5–20mg can also be injected into the arthritis area. Oral prednisone also has rapid effects but is prone to recurrence after discontinuation. Long-term hormone use may lead to complications such as diabetes and hypertension, so it should be avoided if possible.
(2) Treatment during the intercritical and chronic phases In order to prevent acute attacks of pain wind and avoid various complications, active treatment is still necessary during this stage.
1. General Management: Dietary control is crucial, avoiding high-purine foods. Organ meats, bone marrow, seafood, clams, crabs, etc., are the richest in purines; fish, shrimp, meat, peas, spinach, etc., also contain a certain amount of purines; vegetables, fruits, milk, eggs, etc., contain no purines. Obese patients must reduce calorie intake to lower body weight. Drinking plenty of water is recommended to facilitate uric acid excretion. Avoid triggers such as overexertion, stress, alcohol consumption, exposure to cold or dampness, and joint injuries.
2. Use of Hypouricemic Drugs: Indications include patients whose serum uric acid levels remain above 7–8 mg/dl despite dietary control; those with more than two acute attacks per year; those with radiographic evidence of tophi or urate deposits; and those with kidney stones or renal impairment. Maintaining serum uric acid at normal or near-normal levels can prevent acute gout attacks, reduce tophi formation, and mitigate kidney damage. Antihyperuricemic treatments include drugs that promote uric acid excretion and those that inhibit uric acid synthesis. Neither group has anti-inflammatory or analgesic effects, and both may mobilize uric acid into the bloodstream, potentially triggering acute arthritis. Therefore, they should not be used during acute episodes. The choice between the two groups depends on renal function and 24-hour urinary uric acid excretion. Patients with daily uric acid excretion below 600 mg and normal renal function may use uricosuric drugs. Those with impaired renal function or daily uric acid excretion above 600 mg should use uric acid synthesis inhibitors. In cases of markedly elevated serum uric acid or extensive tophi, combining both drugs can accelerate uric acid reduction and tophi resolution.
Currently, the following three uricosuric drugs are commonly used:
(1) Probenecid: Primarily inhibits renal tubular reabsorption of uric acid, promoting excretion. To avoid kidney damage or stone formation due to excessive uric acid excretion, start with a low dose (0.25 g twice daily) and gradually increase to 0.5 g three times daily within two weeks. The maximum daily dose should not exceed 2 g. Side effects (occurring in ~5% of patients) include rash, fever, gastrointestinal irritation, renal colic, and acute gout attacks.
(2) Sulfinpyrazone: A derivative of phenylbutazone, it inhibits renal tubular reabsorption of uric acid and is more potent than probenecid. Start with 50 mg twice daily, gradually increasing to 100 mg three times daily. The maximum daily dose is 600 mg. Combined use with probenecid enhances efficacy. Yaodui It may irritate the gastric mucosa; use with caution in ulcer patients.
(3) Benzbromarone: A potent uricosuric agent widely used in Europe for years. The dose is 25–100 mg once daily, with minimal toxicity and no impact on liver or kidney function. Side effects are rare (rash, fever) but may include gastrointestinal discomfort, renal colic, or acute arthritis flare-ups.
During uricosuric therapy, oral sodium bicarbonate (3–6 g daily) is recommended to alkalinize urine, along with ample fluid intake to maintain a daily urine output of over 2000 ml, facilitating uric acid excretion.
To date, the only drug that inhibits uric acid synthesis is allopurinol. This medication suppresses xanthine oxidase, preventing hypoxanthine and xanthine from converting into uric acid. It gradually oxidizes in the body, forming oxipurinol, which is highly water-soluble and excreted in urine. In the presence of PRPP, it can also be converted into the corresponding nucleotide, depleting PRPP and inhibiting PRPPAT, thereby reducing IMP synthesis. As a result, it rapidly lowers hematuria acid levels, inhibits the formation of pain wind stones and renal uric acid stones, and promotes the dissolution of pain wind stones. The dosage is 100mg three times daily, which can be increased to 200mg three times daily. Combining it with uricosuric drugs may enhance efficacy, though such combinations are generally unnecessary. Some patients may experience side effects such as fever, allergic rashes, abdominal pain, diarrhea, leukopenia, thrombocytopenia, or even liver function impairment. These usually resolve after discontinuation and appropriate treatment. In rare cases, necrotizing dermatitis may occur, which is severe and requires immediate emergency treatment. During medication, transfer pain wind attacks may also occur, which can be managed with adjunctive colchicine therapy.
3. Application of Colchicine In patients with recurrent gout, chronic inflammation is difficult to control. Although the aforementioned treatments are administered, local joint pain or acute attacks may still occur occasionally. In such cases, a small dose of colchicine can be used for maintenance. A daily dose of 0.5mg or 1mg is often sufficient to control symptoms. However, attention should be paid to colchicine's suppression of bone marrow and its potential damage to liver and kidney function.
4. Others For patients with concurrent or complicating conditions such as hypertension, coronary heart disease, obesity, urinary tract infections, or renal failure, symptomatic treatment is necessary. Patients with joint mobility difficulties should undergo physical therapy and exercise. For cases where tophi rupture and form fistulas, surgical curettage should be performed.
(3) Treatment of Asymptomatic Hyperuricemia Opinions vary among experts. Generally, it is believed that patients with urate levels below 8–9mg/dl do not require drug therapy. However, they should avoid overeating (especially high-purine diets), excessive alcohol consumption, overwork, trauma, and stress—factors that may trigger acute attacks. Patients with excessively high uric acid levels should be treated with allopurinol.
(4) Treatment of Secondary Gout In addition to treating the primary disease, the principles for managing gout are the same as described earlier. Allopurinol is the preferred treatment for lowering uric acid levels. Since uric acid production and excretion are higher in these cases, uricosuric drugs may increase the burden on the kidneys and are therefore not recommended.
Due to the diverse manifestations of this disease and sometimes atypical symptoms, the following differential diagnoses should be considered.
(1) Rheumatoid arthritis: More common in young and middle-aged women, often affecting small finger joints, wrists, knees, ankles, sacroiliac joints, and the spine. It presents as migratory symmetric polyarthritis, which can lead to joint stiffness and deformity. It involves recurrent acute episodes on a chronic sexually transmitted disease basis, easily confused with pain wind. However, hematuria acid levels are not elevated, and most rheumatoid factors are positive. X-rays show rough joint surfaces, narrowed joint spaces, or even joint fusion, which is distinctly different from the bone defects seen in pain wind.
(2) Suppurative arthritis and traumatic arthritis: Pain wind in its early stages is often confused with suppurative arthritis or traumatic arthritis. However, the latter two conditions do not exhibit elevated hematuria acid levels, and synovial fluid tests show no urate crystals. Traumatic arthritis usually has a significant injury history, while suppurative arthritis synovial fluid contains large numbers of white blood cells, and cultures may reveal pathogenic bacteria, aiding differentiation.
(3) Cellulitis: During acute pain wind episodes, the soft tissues around the joint often show significant redness and swelling. If joint symptoms are overlooked, it can easily be misdiagnosed as cellulitis. The latter, however, does not involve elevated hematuria acid levels, and systemic symptoms such as fear of cold fever and leukocytosis are more prominent, while joint pain is often less noticeable. Careful differentiation makes diagnosis straightforward.
(4) Pseudopain wind: Caused by calcification of joint cartilage, mostly seen in the elderly, with the knee joint most commonly affected. Acute episodes closely resemble pain wind, but hematuria acid levels are not elevated. Synovial fluid examination reveals calcium pyrophosphate crystals or apatite, and X-rays show cartilage calcification.
(5) Psoriatic arthritis: Often asymmetrically affects distal interphalangeal joints, accompanied by joint destruction and deformity, widened joint spaces, and bone resorption at the tips of toes (fingers). The sacroiliac joints are also frequently involved. Clinically, it resembles rheumatoid arthritis, with about 20% of cases showing elevated hematuria acid levels, making it difficult to distinguish from pain wind.
(6) Other types of arthritis: In the acute phase, differentiation is required from lupus erythematosus, recurrent arthritis, and Reiter syndrome. In the chronic phase, it must be distinguished from hypertrophic arthropathy, traumatic arthritis, and sequelae of suppurative arthritis. Hematuria acid testing aids in diagnosis.






