| disease | Ascending Aortic Aneurysm |
Most ascending aortic aneurysms are caused by cystic degeneration of the middle layer of the aortic wall. Patients are mostly young or middle-aged and often accompanied by dilation of the aortic sinus and valve ring. In severe cases of dilation, the aortic valve leaflets cannot close properly during diastole, resulting in aortic valve insufficiency. However, the aortic valve leaflets themselves show no significant pathology. Some patients may exhibit signs of Marfan syndrome, such as a long head, high-arched palate, slender trunk, limbs, and fingers, joint hyperextension, pigeon breast or funnel chest deformity, and congenital lens dislocation. Other causes of ascending aortic aneurysms include atherosclerosis, syphilitic aortitis, and chest trauma.
bubble_chart Pathological Changes
The vast majority of stirred pulse tumors caused by middle cystic degeneration are fusiform stirred pulse tumors. The affected segment of the ascending stirred pulse expands circumferentially, with the proximal end potentially involving the stirred pulse valve ring, leading to stirred pulse valve insufficiency; the distal end mostly terminates below the origin of the innominate stirred pulse. The elastic layer muscle cells of the stirred pulse wall undergo necrosis and disappear, often presenting cystic spaces containing mucoid material. The inner membrane may exhibit localized tears or develop into dissecting stirred pulse tumors. In rare cases, syphilitic ascending stirred pulse stirred pulse tumors may present as sac-like structures, where the stirred pulse tumor protrudes from a locally weakened area of the stirred pulse wall, typically with a small neck corresponding to the small rupture in the stirred pulse wall.
bubble_chart Clinical ManifestationsTumors that do not invade the aortic valve annulus may not present clinical symptoms in the early stages. If the tumor grows large enough to compress the superior vena cava or innominate vein, it can cause distension and enlargement of the neck and upper limb veins. In advanced cases, if the tumor grows into the anterior chest wall and erodes the sternum, it may cause severe pain or even protrude through the chest wall, presenting as a pulsatile mass. Aortic tumor diseases that lead to dilation of the aortic valve annulus and aortic valve insufficiency will clinically manifest symptoms of congestive heart failure. Physical examination may reveal a diastolic murmur caused by aortic valve insufficiency, widened pulse pressure, and water-hammer pulse. Chest X-rays may show dilation of the ascending aorta and left ventricle. Electrocardiograms often indicate left ventricular hypertrophy and strain. Aortography reveals dilation of the ascending aorta and aortic sinuses. In cases of ascending aortic aneurysms caused by cystic medial degeneration, the lesion is mostly confined to the ascending aorta, with the external diameter of the aorta returning to normal beyond the origin of the innominate artery. If aortic valve insufficiency is present, contrast medium may reflux into the left ventricle during diastole, and the severity of the insufficiency can be assessed based on the amount of reflux.
bubble_chart Treatment Measures
Ascending aortic aneurysm tumor diseases For example, most patients are diagnosed after presenting symptoms or signs of aortic valve insufficiency. Aortic valve insufficiency often worsens gradually as the aortic aneurysm enlarges. Therefore, surgical treatment should be performed as early as possible after diagnosis. For cases without aortic valve insufficiency, surgical treatment should also be considered to prevent aneurysm rupture or the complication of aortic dissection. The vast majority of ascending aortic aneurysms are fusiform aneurysms. The treatment principle is to resect the diseased segment of the ascending aorta and replace it with an artificial blood vessel (Figure 1) or a homologous aorta. Homologous aortas are scarce in supply and may undergo degenerative changes over time, affecting treatment efficacy, so they are rarely used nowadays. For patients with aortic valve insufficiency, aortic valve replacement surgery is often required simultaneously. Since the procedure involves blocking blood flow in the ascending aorta, care must be taken to protect the heart, brain, spinal cord, and visceral organs from ischemic and hypoxic damage, and to prevent the left ventricle from acute dilation and failure due to obstructed blood ejection.
 |  |  |
| (1) | (2) | (3) |
Figure 1 Ascending aortic aneurysm
replaced with an artificial blood vessel connecting the proximal and distal incisions
Operative Technique: A midline incision is made in the anterior chest, and the sternum is longitudinally split. Cannulas for venous drainage are inserted into the superior and inferior vena cava via the right atrium and right atrial appendage, respectively, or a single venous drainage cannula is placed in the right atrium. The arterial perfusion cannula is inserted into the femoral artery. A decompression drainage catheter is placed in the left ventricle through a left atriotomy along the interatrial groove or via the right superior pulmonary vein. After initiating cardiopulmonary bypass, the body temperature is lowered to approximately 25°C. Ice-cold saline is infused into the pericardial cavity for local profound hypothermia. The distal ascending aorta between the aneurysm and the innominate artery is dissected and clamped. The anterior wall of the aortic aneurysm is longitudinally incised, and catheters are placed into the openings of the left and right coronary arteries to inject cold cardioplegic solution under pressure. The ascending aorta is transected proximal and distal to the aneurysm, with the proximal incision made at least 5 mm above the coronary artery orifices. A Dacron or Gore-Tex graft of appropriate length and diameter is anastomosed end-to-end to the distal and proximal ends of the ascending aorta. If a Dacron graft is used, it should be preclotted to prevent bleeding. Two stay sutures are placed on either side of the anastomosis for traction and fixation. The posterior wall of the anastomosis is then continuously sutured in a full-thickness fashion using 2-0 Dacron sutures, with stitches placed approximately 3 mm from the aortic edge and spaced 2–3 mm apart. After completing the posterior wall anastomosis, the suture ends are tied externally to the stay sutures on the aortic wall. The anterior wall is then continuously sutured in a similar fashion. Alternatively, the posterior wall of the aneurysm may be left intact after incising the aneurysm, and the graft can be placed inside the aneurysm cavity for anastomosis. After completing the anastomosis, fluid is injected into the graft to check for leaks, and additional sutures are placed if necessary. Residual air is evacuated from the graft before slowly releasing the aortic clamp. Rewarming is initiated via cardiopulmonary bypass, and bypass is discontinued once the body temperature reaches above 35°C and the heart resumes strong contractions. The excised aortic aneurysm wall may be wrapped around the graft, and its edges sutured to reinforce and achieve hemostasis (Figure 2).
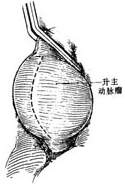
(1) Incision
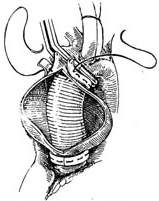
(2) Open the stirred pulse tumor, preserve the posterior wall, and anastomose the upper and lower incisions with an artificial blood vessel

(3) Suture the stirred pulse tumor wall over the artificial blood vessel externally
Figure 2: Ascending aortic stirred pulse tumor—Anastomosis performed by placing an artificial blood vessel into the stirred pulse tumor cavity
For ascending aortic stirred pulse tumors with aortic valve insufficiency, it is often necessary to remove the stirred pulse tumor and the aortic valve, followed by aortic valve replacement and stirred pulse tumor resection with artificial blood vessel transplantation. This procedure is relatively complex and technically challenging, and three methods can be adopted.
1. Simultaneous separate aortic valve replacement and ascending aortic stirred pulse tumor resection with artificial blood vessel transplantation. This is suitable for cases where the aortic valve sinus is not enlarged and the coronary artery orifice has not shifted upward.
Surgical technique: The operation must be performed under cardiopulmonary bypass combined with moderate hypothermia and myocardial protection measures. Blood is supplied via cannulation of the common femoral artery. The distal segment of the ascending aorta is clamped, and the anterior wall of the stirred pulse tumor is longitudinally incised. The aortic valve leaflets are excised, and the artificial aortic valve is sutured and fixed to the aortic valve annulus. The ascending aorta is then transected horizontally at least 5 mm away from the coronary artery orifice. A segment of artificial blood vessel is anastomosed end-to-end to the proximal and distal cut ends of the ascending aorta. After completing the artificial blood vessel transplantation, the stirred pulse tumor wall can be wrapped around to reinforce the artificial blood vessel (Figure 3).
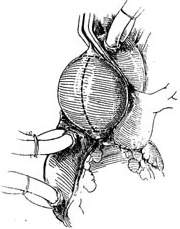 |  |
(1) Incision | (2) Aortic valve implantation |
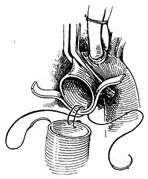 |  |
(3) Artificial blood vessel implantation | (4) Artificial blood vessel implantation |
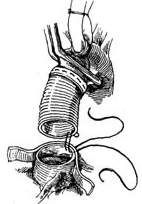 | |
(5) Artificial blood vessel implantation | |
Figure 3: Aortic valve and artificial blood vessel implantation after ascending aortic stirred pulse tumor resection
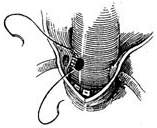
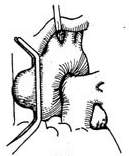
2. Ascending aortic aneurysm with aortic valve replacement and prosthetic graft transplantation This procedure is suitable for cases where the aortic sinus is enlarged and the coronary artery ostia are displaced upward. Under cardiopulmonary bypass combined with hypothermia and myocardial protection measures, the ascending aorta is clamped distal to the aneurysm. The anterior wall of the aortic aneurysm is longitudinally incised, and the aortic valve is excised. A pre-clotted prosthetic graft with an aortic valve of appropriate size is selected. The prosthetic graft with the valve is first sutured to the aortic valve annulus using interrupted mattress sutures or continuous sutures with pledgets. The suture intervals should be tight to prevent bleeding. The left coronary artery ostium and the adjacent aortic wall are excised, and a small hole with a diameter of about 8–10 mm is made in the corresponding area of the prosthetic graft using an electrocautery knife. The left coronary artery ostium is anastomosed to the small hole in the prosthetic graft with a 4-0 Prolene continuous suture. The right coronary artery ostium and the adjacent aortic wall are then excised and similarly anastomosed to another small hole created in the corresponding area of the prosthetic graft. Finally, an end-to-end anastomosis is performed between the prosthetic graft and the distal ascending aorta (Figure 4).
 |  |
| (1) | (2) |
 | 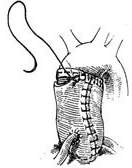 |
| (3) | (4) |
Figure 4 Treatment of ascending aortic aneurysm using a valved artificial vessel
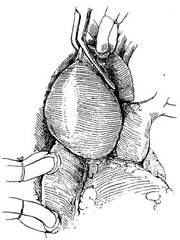
3. Resection of Ascending Aortic Saccular Aneurysm Since resection of an ascending aortic saccular aneurysm does not require blocking the ascending aortic blood flow, extracorporeal circulation is unnecessary. A midline incision is made in the chest, the sternum is longitudinally sawed open, the pleura is pushed aside, the pericardium is incised, and the aneurysm is exposed and dissected. After isolating the aneurysm, a non-traumatic vascular clamp is placed near the aortic wall at the base of the aneurysm. Using pledgeted sutures, a full-thickness interlocking mattress suture is first applied to the aortic wall below the clamp. The aneurysm is then excised proximal to the vascular clamp, followed by a continuous suture layer (Figure 5).
(1) A non-traumatic vascular clamp is placed near the aortic wall at the base of the aneurysm
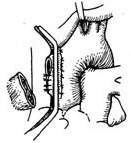
(2) Using pledgeted sutures, a full-thickness interlocking mattress suture is first applied to the aortic wall below the clamp. The aneurysm is then excised proximal to the vascular clamp, followed by a suture layer
Figure 5 Resection of ascending aortic saccular aneurysm
Treatment Outcomes: The surgical mortality rate for ascending aortic aneurysms has decreased to 5–10%. Cases caused by syphilitic aortitis or complicated by dissecting aneurysms have a higher early mortality rate. Among postoperative survivors, 90% experience symptom resolution or significant relief, with cardiac function recovering to Class I–II.





