| disease | Double Outlet Right Ventricle |
Double outlet right ventricle means that both the aorta and the pulmonary artery originate from the right ventricle, or one major artery and most of the other major artery originate from the right ventricle, with the ventricular septal defect being the only outlet for the left ventricle. The ventricular septal defect is usually larger than the aortic orifice, and only in 10% of cases is the ventricular septal defect smaller than the aortic opening. Approximately 60% of ventricular septal defects are located below the aortic valve, 30% below the pulmonary valve, and in a few cases, the defect is positioned between the aortic and pulmonary valve openings. Rarely, the ventricular septal defect is located in the mid-to-lower part of the ventricular septum, far from the major artery openings. Regarding the position of the major arteries: the most common arrangement is the aorta and pulmonary artery openings lying side by side on the same plane, with the aorta on the right. The next most common is the aortic opening positioned to the right and posterior to the pulmonary artery opening, or to the right and anterior. The aortic opening located to the left and anterior of the pulmonary artery opening is more commonly seen in cases of double outlet right ventricle with atrioventricular discordance. Atrioventricular connection: In 90% of cases, the atrioventricular relationship is concordant, with the right atrium connecting to the right ventricle and the left atrium to the left ventricle. Discordant atrioventricular connections account for only about 10%. Other associated anomalies include pulmonary valve or infundibular stenosis, subaortic stenosis, atrioventricular valve malformations, ventricular hypoplasia, atrial septal defects, and abnormal coronary artery openings.
bubble_chart Pathological ChangesThe hemodynamic changes in double outlet right ventricle primarily depend on the location and size of the ventricular septal defect (VSD), as well as the presence and degree of associated pulmonary stenosis. When the VSD is located below the aortic valve without pulmonary stenosis, most of the left ventricular blood flows directly through the defect into the aorta, while the right ventricular blood primarily enters the pulmonary artery, leading to increased pulmonary blood flow. Clinically, this resembles a simple VSD with pulmonary hypertension. When the VSD is located below the pulmonary valve without pulmonary stenosis, the left ventricular blood mainly flows directly through the defect into the pulmonary artery, while the right ventricular blood primarily enters the aorta. Clinically, this resembles complete transposition of the great arteries with VSD, presenting with pulmonary congestion and severe cyanosis. If the VSD is large, left ventricular ejection is unimpeded, and the pressures in the left and right ventricles are equal. If the VSD is small, left ventricular ejection is obstructed, creating a pressure gradient between the left and right ventricles, with the left ventricular pressure being higher than the right. Regardless of the VSD's location and size, if pulmonary stenosis is present, the clinical presentation resembles severe tetralogy of Fallot, with pulmonary ischemia and severe cyanosis.
bubble_chart Clinical Manifestations
There are many classification methods for double outlet right ventricle. From the perspective of surgical treatment, Lev, Kirklin, and others classified it based on the anatomical relationship between the ventricular septal defect and the great arteries as follows (Figure 1):
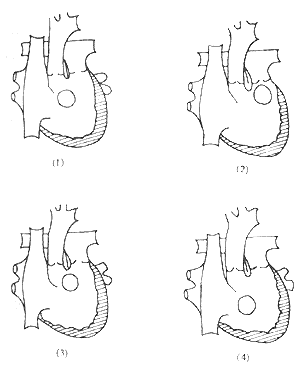
Figure 1 Schematic diagram of anatomical malformation classification
(1) Subaortic ventricular septal defect; (2) Subpulmonary ventricular septal defect; (3) Ventricular septal defect related to both great arteries; (4) Ventricular septal defect unrelated to both great arteries
I. Double outlet right ventricle with concordant atrioventricular relationship
1. Subaortic ventricular septal defect
With pulmonary stenosis
Without pulmonary stenosis
2. Subpulmonary ventricular septal defect
With pulmonary stenosis
Without pulmonary stenosis
3. Ventricular septal defect related to both great arteries
With pulmonary stenosis
Without pulmonary stenosis
4. Ventricular septal defect unrelated to both great arteries
With pulmonary stenosis
Without pulmonary stenosis
II. Double outlet right ventricle with discordant atrioventricular relationship
1. Subaortic ventricular septal defect
With or without pulmonary stenosis
2. Subpulmonary ventricular septal defect
With or without pulmonary stenosis
3. Ventricular septal defect related to both great arteries
With or without pulmonary stenosis
4. Ventricular septal defect unrelated to both great arteries
With or without pulmonary stenosis
III. Other complex types
Associated with total anomalous pulmonary venous drainage, complete common atrioventricular canal, mitral atresia or stenosis, aortic stenosis or hypoplasia.
[Clinical manifestations] The clinical manifestations of double outlet right ventricle are diverse and vary depending on the type of lesion, the size of the ventricular septal defect and its relationship with the aorta and pulmonary artery, the direction of left ventricular blood flow through the ventricular septal defect, pulmonary blood flow, and the presence of other cardiac anomalies. However, regardless of the type of lesion, symptoms typically appear early in life (average 2 months, range from 1 day to 4 years), with the most common being cyanosis and congestive heart failure. Severely affected newborns often die early without treatment. The surgical mortality rate for corrective surgery performed within the first 2 months of life was once as high as 50%, necessitating palliative procedures such as pulmonary artery banding or systemic-to-pulmonary shunts to prolong life. In recent years, the surgical mortality rate for corrective surgery in children around 2 years of age has decreased to approximately 10%. Chest X-ray, electrocardiogram, and cardiac catheterization findings vary significantly depending on the type of lesion. Two-dimensional echocardiography and cineangiocardiography are the most reliable diagnostic methods, both capable of demonstrating the anterior displacement of the aorta and the common origin of the great arteries from the right ventricle, the absence of continuity between the base of the anterior mitral leaflet and the aortic semilunar valve, and the relative positions of the aortic and pulmonary artery orifices, the location and size of the ventricular septal defect, and associated cardiac anomalies such as pulmonary stenosis or cleft anterior mitral leaflet.
(2) Double outlet right ventricle, atrioventricular concordance, dextroposition of the main stirred pulse, sub-stirred pulse valvular ventricular septal defect with pulmonary stirred pulse stenosis. The clinical manifestations are similar to severe tetralogy of Fallot, including cyanosis, squatting, clubbing of fingers (toes), and hypoxic spells. Chest X-ray: shows pulmonary ischemia. Electrocardiogram: reveals left and right atrial enlargement and right ventricular hypertrophy. Right heart catheterization: The characteristic finding is that the oxygen saturation in the right ventricle is higher than in the right atrium due to left ventricular blood ejection through the ventricular septal defect into the right ventricle and then into the main stirred pulse. Selective right ventricular angiography: shows simultaneous opacification of the right ventricle, main stirred pulse, and pulmonary stirred pulse, along with stenosis of the right ventricular fistula disease infundibulum and/or pulmonary stirred pulse.
(3) Double outlet right ventricle, atrioventricular concordance, dextroposition of the aorta with subpulmonary ventricular septal defect, with or without pulmonary stenosis. Clinical manifestations include cyanosis, dyspnea, and congestive heart failure in infancy, as well as growth retardation and clubbing of fingers/toes. Chest X-ray: shows pulmonary congestion and cardiomegaly. ECG: right axis deviation and right ventricular hypertrophy. Right heart catheterization: pressures in the left and right ventricles are similar to those in the aorta and pulmonary artery, with stepwise increases in oxygen saturation from the right atrium to the right ventricle and pulmonary artery. Selective right ventriculography: simultaneous opacification of the right ventricle, aorta, and pulmonary artery.
(4) Double outlet right ventricle, atrioventricular concordance with ventricular septal defect related to both great arteries. The aorta and pulmonary artery are side by side, with a large ventricular septal defect located beneath the openings of both great arteries. Clinical manifestations are similar to those of subaortic ventricular septal defect, with a large shunt leading to grade I cyanosis or heart failure. Chest X-ray: increased pulmonary blood flow and cardiomegaly. ECG: biventricular hypertrophy. Right heart catheterization: right ventricular pressure is similar to systemic arterial pressure, with elevated oxygen saturation in the right ventricle. Selective right ventriculography: simultaneous opacification of the aorta and pulmonary artery, with the ventricular septal defect located beneath both great arteries.
(5) Double outlet right ventricle, atrioventricular concordance with ventricular septal defect unrelated to the great arteries. The aorta and pulmonary artery are side by side, with the ventricular septal defect located below the conus, beneath the septal leaflet of the tricuspid valve (atrioventricular canal type), or within the apical trabeculations. Clinical manifestations resemble those of a large ventricular septal defect with pulmonary hypertension. Chest X-ray: pulmonary congestion and cardiomegaly. ECG: biventricular hypertrophy. Right heart catheterization: elevated oxygen saturation in the right ventricle. Selective right ventriculography: simultaneous opacification of both great arteries and visualization of the ventricular septal defect location.
(6) Double outlet right ventricle, atrioventricular discordance, often accompanied by pulmonary stenosis and dextrocardia, with the ventricular septal defect usually located below the pulmonary valve. Clinical manifestations include cyanosis and hypoxia in infancy. Chest X-ray: shows normal or reversed situs of the heart and viscera. ECG: biventricular hypertrophy. Right heart catheterization and ventriculography: pressures in the left and right ventricles are similar, catheter insertion into the pulmonary artery is difficult, pulmonary artery oxygen saturation is elevated but pressure is reduced. Angiography reveals both great arteries arising from the right ventricle, with the ventricular septal defect located below the crista supraventricularis and pulmonary valve stenosis.
bubble_chart Treatment Measures
Corresponding to the classification of clinical manifestations:
(1) Treatment: Under extracorporeal circulation combined with low warm purgation, the right ventricle is incised to create an intraventricular tunnel. Specifically, a Dacron fabric patch is used to construct a tunnel between the ventricular septal defect and the main stirred pulse, directing left ventricular blood through the ventricular septal defect and tunnel into the main stirred pulse.
(2) Treatment: Under extracorporeal circulation combined with low warm purgation, the right ventricle is incised, and the hypertrophic muscle of the fistula disease infundibulum is resected. The fused commissures of the pulmonary stirred pulse valve membrane are incised. If the valve annulus is narrow, a patch is applied to the right ventricular outflow tract or across the pulmonary stirred pulse valve annulus to reconstruct and enlarge the right ventricular outflow tract. Simultaneously, a tunnel is created within the right ventricle to connect the ventricular septal defect with the main stirred pulse.
(3) Treatment: Under extracorporeal circulation combined with low warm purgation, the right ventricle is incised, and a tunnel is created within the right ventricle between the ventricular septal defect and the pulmonary stirred pulse, connecting the pulmonary stirred pulse to the left ventricle. This results in a physiological transposition of the great stirred pulses. Subsequently, a diversion procedure (Mustard or Senning operation) is performed in the right atrium. Alternatively, another surgical method involves creating a tunnel within the right ventricle to connect the ventricular septal defect with the main stirred pulse, allowing left ventricular blood to eject into the main stirred pulse and right ventricular blood into the pulmonary stirred pulse. If the tunnel causes obstruction of the right ventricular outflow tract, a valved extracardiac conduit is used to establish a pathway between the right ventricle and the pulmonary stirred pulse. If pulmonary stirred pulse stenosis is present, simultaneous enlargement of the right ventricular outflow tract is required.
(4) Treatment: Under extracorporeal circulation combined with low warm purgation, the right ventricle is incised, and an oval-shaped patch is used to connect the ventricular septal defect with the main stirred pulse, creating an internal tunnel between the left ventricle and the main stirred pulse.
(5) Treatment: Under extracorporeal circulation combined with low warm purgation, the right ventricle is incised, followed by:
1. For ventricular septal defects located beneath the septal leaflet of the tricuspid valve, small defects require enlargement. Subsequently, an intraventricular tunnel repair is performed to connect the ventricular septal defect to the main stirred pulse, closing the pulmonary stirred pulse orifice. A valved extracardiac conduit is used to establish a pathway between the right ventricle and the pulmonary stirred pulse.
2. For ventricular septal defects located beneath the septal leaflet of the tricuspid valve accompanied by pulmonary stirred pulse stenosis, an intraventricular tunnel repair is performed to connect the left ventricle to the main stirred pulse. A pericardial or Dacron patch is used for right ventricular outflow tract reconstruction, including transannular patch plasty across the pulmonary stirred pulse valve annulus.
(6) Treatment: Under extracorporeal circulation combined with low warm purgation, an intracardiac tunnel procedure is performed:
1. Intracardiac tunnel repair: Through a morphologically right ventricular incision on the left side, the hypertrophic muscle bundles below the pulmonary stirred pulse are resected, and the pulmonary stirred pulse valve is incised to relieve stenosis. A patch is used to repair the ventricular septal defect and connect it to the pulmonary stirred pulse, ensuring the pulmonary stirred pulse outlet originates from the morphologically left ventricle.
2. Intracardiac tunnel repair with valved extracardiac conduit correction: Through an incision in the morphologically left ventricle, the ventricular septal defect is closed to direct systemic ventricular blood flow into the main stirred pulse. The main pulmonary stirred pulse is transected, with the proximal end sutured closed and the distal end connected to the right ventricular incision via a valved extracardiac conduit, directing systemic venous blood into the pulmonary stirred pulse.
There are two main types of valved extracardiac conduits for reconstructing the right ventricle-pulmonary stirred pulse pathway: the homograft aortic conduit with an aortic valve and the Dacron conduit with a heterograft (porcine) valve. The latter is preferable to the former due to the following advantages:
(1) Dacron conduits are readily available in various sizes and are easier to preserve and transplant.
(2) Post-transplantation, the transvalvular pressure gradient is smaller.
(3) Homograft aortic conduits are prone to degenerative changes, leading to calcification, stenosis, and eventual failure.
The surgical mortality rate for double outlet right ventricle remains relatively high, primarily due to severe pulmonary vascular obstructive disease, inadequate relief of pulmonary stenosis, unsatisfactory correction or poor management of associated malformations with significant hemodynamic impact, and complications such as complete atrioventricular block leading to low cardiac output syndrome. Therefore, it is considered that in patients with double outlet right ventricle, if the pulmonary vascular resistance exceeds 800 dyn·s·cm⁻⁵ and the pulmonary-to-systemic blood flow ratio is less than 1:3, or in patients with pulmonary stenosis, if the ratio of right ventricular pressure to left ventricular pressure measured at the end of surgery is greater than 0.75, the surgical mortality rate is high. Common causes of death include heart failure, low cardiac output syndrome, hemorrhagic pulmonary edema, arrhythmias, complete atrioventricular block, respiratory failure, and infections.





