| disease | Cardiac Cancer |
| alias | Malignant Neoplasm of Cardia |
The incidence of cardia cancer is also high in the high-risk areas of esophageal cancer in our country. According to statistics from these regions and cancer research institutions, the ratio of esophageal cancer to cardia cancer is approximately 2:1. The correct definition of cardia cancer is adenocarcinoma occurring in the gastric cardia, which is within about 2cm below the esophagogastric junction. It is a special type of stomach cancer and should be distinguished from cancer of the lower esophagus. However, it is different from stomach cancer in other parts, with its own anatomical and histological characteristics, clinical manifestations, unique diagnostic and treatment methods, and relatively poor surgical treatment outcomes.
bubble_chart Pathological Changes
(1) General Classification
1. Advanced Stage The gastrointestinal classification generally follows the Borrman classification, which includes the basic categories of fungating, ulcer type I, ulcer type II, and infiltrative types. Based on this, Chinese authors have classified cardiac cancer into four types. ① Protruding type: The tumor is a clearly edged mass protruding into the lumen, resembling a cauliflower, nodular mass, or polyp, and may have a shallow ulcer; ② Localized ulcer type: The tumor is a deep ulcer with raised edges like a dike, and the cut surface is clearly demarcated from normal tissue; ③ Infiltrative ulcer type: The edges of the ulcer are unclear, and the cut surface is indistinct from surrounding tissues; ④ Infiltrative type: The tumor grows infiltratively within the cardiac wall, with uniform thickening of the affected area, no clear boundaries with surrounding tissues, and the surrounding mucous membrane often shows radial contraction.
The general classification is related to histological types, with types ① and ② mostly being well-differentiated adenocarcinoma and mucinous adenocarcinoma. The proportion of poorly differentiated adenocarcinoma and mucinous adenocarcinoma increases in the infiltrative ulcer type. The infiltrative type is mostly poorly differentiated diffuse adenocarcinoma or mucinous adenocarcinoma. The surgical prognosis is best for the protruding type, followed by the localized ulcer type, with the infiltrative ulcer type being poorer, and the infiltrative type being the worst.The histological types of cardiac adenocarcinoma mainly include two categories: adenocarcinoma and mucinous adenocarcinoma with significant mucus secretion. These two categories are further divided into well-differentiated, poorly differentiated, and diffuse subtypes based on the degree of differentiation. The degree of differentiation is closely related to surgical prognosis. Besides adenocarcinoma and mucinous adenocarcinoma, cardiac cancer also has some rare histological types, such as adenosquamous carcinoma, undifferentiated carcinoma, carcinoid (argentaffin cell carcinoma), and carcinosarcoma.
2. Early Stage The gross morphology of early cardiac cancer is similar to early cancers in other parts of the stomach and esophagus. It can be simply divided into three types: ① Depressed type: The cancerous mucous membrane shows irregular grade I depression, with a few being shallow ulcers, and the boundary with the surrounding normal mucous membrane is unclear, often with poor differentiation under the microscope; ② Protruding type: The cancerous mucous membrane is thickened and rough, slightly raised, with some showing patches, nodules, or polypoid shapes, mostly well-differentiated adenocarcinoma; ③ Latent type: The mucous membrane of the lesion is slightly darker in color and coarser in texture, with no significant gross changes, and is diagnosed only after histological examination, being the earliest form among the three types.
(2) Histogenesis of Cardiac CancerIn the past, gastric ulcer, gastric polyp (adenoma), and chronic atrophic gastritis were considered precancerous lesions of stomach cancer. Recent studies have found that the chance of cancerization in these conditions is very small. Especially in the cardiac region, these three conditions occur even less frequently than in other parts of the stomach. Therefore, they are clearly not closely related to the histogenesis of cardiac cancer.
Currently, a more accepted view is that cardiac cancer originates from the neck stem cells of the cardiac glands, which have the potential for multidirectional differentiation and can form adenocarcinoma with characteristics of cardiac or glandular epithelium. Most cardiac cancers are found to be mixed types under light microscopy, electron microscopy, and histochemical studies, strongly supporting this view. Atypical hyperplasia is a precancerous lesion of cardiac cancer, and it is also a key pathological process shared by ulcer, polyp, and atrophic gastritis related to the onset of cardiac cancer. Only when they undergo atypical hyperplasia changes can they become cancerous, with most colonic-type metaplasia having the nature of atypical hyperplasia.
Clinical Pathological Staging of Cardiac Cancer
The TNM staging system for stomach cancer revised by the International Union Against Cancer (UICC) in 1987 is as follows (Table 1):
Table 1 TNM Staging of Stomach Cancer
| Stage 0 | Tis | N | 0M | 0
| Stage IA | T1 | N | 0M | 0
| Stage IB | T1 | N1 | M | 0
| T2 | N | 0M | 0|
| Stage II | T1 | N2 | M | 0
| T2 | N1 | M | 0|
| T3 | N | 0M | 0|
| Stage IIIA | T2 | N2 | M | 0
| T3 | N1 | M | 0|
| T4 | N | 0M | 0|
| Stage IIIB | T3 | N2 | M | 0
| T4 | N2 | M | 0|
| Stage IV | T4 | N2 | M | 0
| Any T | Any N | M1 |
T refers to the primary tumor: Tis is carcinoma in situ within the epithelium without invasion of the lamina propria, T1 tumor invades the lamina propria or submucosa, T2 tumor invades the muscularis propria and subserosa, T3 tumor penetrates the serosa (visceral peritoneum) without reaching adjacent structures, T4 tumor invades adjacent structures (including spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum).
N refers to regional lymph nodes, N0 indicates no regional lymph node metastasis, N1 indicates metastasis in perigastric lymph nodes within 3cm of the tumor periphery, N2 indicates metastasis in perigastric lymph nodes beyond 3cm of the tumor periphery, or metastasis along the left gastric, common hepatic, splenic, and celiac arteries.
M refers to distant metastasis, M0 indicates no distant metastasis, M1 indicates the presence of distant metastasis. The site of metastasis can also be specified, such as: PUL for lung, OSS for bone, HEP for liver, BRA for brain, PER for peritoneal membrane, etc.
Among the 937 cases of gastric cardia cancer reported by the Cancer Hospital of the Chinese Academy of Medical Sciences, TNM staging data were available for 629 cases, including 152 cases (24.1%) in stage I, 179 cases (28.5%) in stage II, and 298 cases (47.4%) in stage III. It can be seen that 3/4 (75.9%) of the gastric cardia cancer cases seeking treatment at the hospital were in stages II and III, with only about 1/4 of the cases being in an early stage.
bubble_chart Clinical Manifestations
Early-stage cardia cancer patients lack clear characteristic symptoms. Symptoms such as upper abdominal discomfort, grade I postprandial fullness, indigestion, and dull pain in the epigastric region are easily confused with peptic ulcer symptoms, leading patients to overlook them and resort to taking stomach medicine. It is not until the difficulty in swallowing worsens that patients seek medical attention. Another initial symptom of cardia cancer is upper gastrointestinal bleeding, manifested as hematemesis or melena. Depending on the severity of the bleeding, it may be accompanied by collapse, shock, or grade III anemia. The incidence of this condition accounts for about 5% of patients. Due to the lack of choking symptoms, such patients are often misdiagnosed with peptic ulcer bleeding and are operated on by abdominal surgeons, with the diagnosis only confirmed during surgery. Because most of these cases are emergency surgeries, with insufficient preparation in various aspects, the incidence of surgical complications and mortality rates are relatively high, and the treatment outcomes are poor. In advanced-stage cases, besides difficulty in swallowing, persistent dull pain in the upper abdomen and back may occur, indicating that the cancer has involved the pancreas and other retroperitoneal tissues, which is a contraindication for surgery.
Early-stage cardia cancer patients show no positive signs, while mid-advanced stage patients may exhibit anemia, low plasma protein, weight loss, and even dehydration. If abdominal masses, hepatomegaly, signs of ascites, or pelvic masses (on rectal examination) are present, these are indications that surgery is not suitable.
The positive rate of cytological diagnosis for cardia cancer is lower than that for esophageal cancer, which is also due to the conical anatomical characteristics of the cardia that make it difficult for the balloon to come into contact with the tumor. The diagnostic rate has improved after switching to a larger balloon.
X-ray barium contrast is the main method for diagnosing cardia cancer. Early manifestations include subtle mucosal changes, small ulcer niches, and less obvious but constant filling defects. In early cases, fiberoptic gastroscopy combined with cytological brushing and biopsy pathology must be performed to confirm the diagnosis. In advanced stage cases, X-ray findings are clear, including soft tissue shadows, mucosal destruction, ulcers, niches, filling defects, twisted and narrowed cardia passage, invasion of the lower esophagus, and infiltration of the gastric wall with rigidity and reduced gastric volume in both the greater and lesser curvatures of the gastric body.
bubble_chart Treatment Measures
(1) Surgical Indications for Cardiac Cancer
To date, surgical treatment is recognized as the preferred treatment for cardiac cancer. Due to its histological nature as adenocarcinoma or mucinous adenocarcinoma, radiotherapy is almost ineffective, and chemotherapy has limited efficacy. Surgical indications for cardiac cancer include: ① Diagnosis confirmed by X-ray, cytology, and endoscopy; ② Exclusion of lymph node, liver, adrenal gland, omentum, peritoneum, and pelvic metastases by ultrasound, abdominal CT scan, or laparoscopy, with no ascites; ③ Generally in moderate or better condition, without major cardiopulmonary or other organ complications.
Due to the anatomical characteristics of the cardia, which is adjacent to the liver, spleen, transverse colon, pancreatic tail, kidney, adrenal gland, small intestine, diaphragm, and posterior peritoneum, and has rich lymphatic drainage spreading upward into the mediastinum and downward along the greater and lesser curvatures, it can also infiltrate within the gastric wall, potentially involving the entire stomach. Therefore, conventional gastrointestinal imaging cannot fully display all these processes. The use of double-contrast imaging with a foaming agent can clearly show the mass, soft tissue shadows, mucosal destruction, ulcers, and the extent of gastric wall thickening, but X-ray changes are often milder than the actual situation. Abdominal CT can help understand the relationship between the tumor and surrounding organs, but compared to esophageal CT findings, positive findings for cardiac cancer are often less definitive. For example, whether the pancreas is involved is often misjudged; CT may suspect pancreatic tail infiltration when there is no adhesion, or may not detect pancreatic involvement when the tumor is actually adherent to the pancreas. CT is useful for detecting liver metastases but is less accurate for local lymph node metastasis. In summary, preoperative assessment of the extent of cardiac cancer and the feasibility of resection is quite challenging and remains an unresolved clinical issue. To avoid missing treatment opportunities, positive findings from abdominal ultrasound, CT, and esophageal-gastric imaging should prompt exploration unless widespread metastasis is confirmed, aiming to resect the lesion and restore gastrointestinal continuity.
(2) Surgical Approaches and Methods for Cardiac Cancer
The Thoracic Surgery Department of the Cancer Hospital of the Chinese Academy of Medical Sciences commonly uses a left posterolateral standard thoracotomy incision through the 7th rib bed or intercostal space, followed by a radial incision at the top of the left diaphragm centered on the esophagus to open the abdomen. This approach provides good exposure of the cardia, sufficient for subtotal gastrectomy and lymph node dissection around the stomach and left gastric vessels. If extended resection is needed, such as total gastrectomy or combined resection of the spleen and part of the pancreas, the incision can be extended forward and downward to the upper abdominal wall, cutting through the left costal cartilage arch, diaphragm, and abdominal wall muscles, easily converting to a thoracoabdominal incision for full exposure of the upper abdomen.
In patients with low cardiopulmonary reserve and elderly patients, a cervical and abdominal two-incision non-thoracotomy esophageal inversion stripping with partial gastrectomy and cervical esophagogastric anastomosis can be used. After abdominal exploration confirms resectability, an esophageal bougie is passed from the gastric fundus or abdominal esophagus to the neck, where the cervical esophagus is exposed. The esophagus is ligated and fixed to the bougie below the intended anastomosis site, the upper esophagus is cut, and the bougie is pulled steadily and evenly to invert and strip the esophagus from top to bottom. The stomach is freed, and partial gastrectomy is performed, with the greater curvature shaped into a tube, pulled up through the esophageal bed to the neck for anastomosis with the esophagus. The drawback of this procedure is the limited extent of gastric resection, which may result in residual cancer at the gastric margin. When there is a history of mediastinal inflammation, such as lymph node involvement causing adhesions, inversion stripping may be difficult, leading to inability to strip or tearing of the tracheobronchial membrane, requiring immediate thoracotomy for repair. If difficulty is anticipated, thoracotomy for resection is preferable (Figure 1).
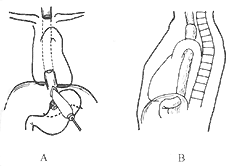
Figure 1 Non-thoracotomy esophageal inversion stripping for cardiac cancer resection and cervical esophagogastric anastomosis
A. Esophageal inversion stripping and partial gastrectomy
B. Cervical esophagogastric anastomosis via the esophageal bed
For patients with insufficient cardiopulmonary function, another surgical approach is the combined median sternotomy and upper abdominal midline incision. During the operation, care should be taken to prevent bilateral pleural membrane rupture, and the pericardium should be freed from the diaphragmatic surface. The diaphragm is incised along the midline to reach the esophageal hiatus, and the pericardium is pulled forward to expose the posterior mediastinum. Then, the cardiac cancer and the lower segment of the esophagus are routinely removed, and the residual stomach is pulled up and anastomosed with the esophagus in the posterior mediastinum. This incision has certain limitations in exposing the posterior mediastinum, and an esophageal-gastric mechanical anastomosis device can be used to ensure the quality of the anastomosis.
The commonly used surgical method is proximal subtotal gastrectomy. It is suitable for tumors in the cardia region that are not large and extend along the lesser curvature not exceeding one-third of its total length. The specific surgical procedure is as follows: a left posterolateral thoracotomy is performed at the 7th intercostal space or rib bed, and the lower segment of the esophagus is explored. Then, the diaphragm is incised anteriorly to the left with the hiatus as the axis, and the abdomen is explored. If there is no liver or abdominal membrane metastasis or extensive lymph node metastasis, the greater omentum, left gastroepiploic membrane stirred pulse, and short gastric vessels in the gastrosplenic ligament are severed along the greater curvature. The left crus of the diaphragm is severed to fully expose the lower segment of the esophagus, and lymph nodes in this area (including those in the inferior pulmonary ligament) are cleared. The pancreas body and tail are exposed with gauze pads, and the left gastric vessels and nearby lymph nodes are carefully cleared. The left gastric vessels are ligated and severed, and the hepatogastric ligament is severed. The proximal stomach is completely freed, and a gastric tube is made on the greater curvature side. If a gastric stapler is available, it can save operation time. The resection margin should be at least 5 cm away from the tumor edge. The gastric tube is rotated 90° clockwise and then end-to-end anastomosed with the lower esophageal stump. The inner layer is a full-thickness interrupted suture, and the outer layer involves invaginating the gastric seromuscular layer around the anastomosis for about 2 cm, resembling a telescope. Before anastomosis, to prevent the appetite mucosa from being too long and covering the muscle layer edge, which may affect the anastomosis, the muscle layer at the gastric tube opening can be circumferentially incised. At this point, the relaxed mucosa will be exposed like a sleeve due to the retraction of the distal muscle layer. Hemostasis is fully performed in the submucosal layer, and the excess mucosa is trimmed at the level of the distal muscle layer. At this point, the mucosa at the gastric tube opening is flush with the muscle layer, providing a clear field of view during anastomosis, which helps in precise alignment (Figure 2).
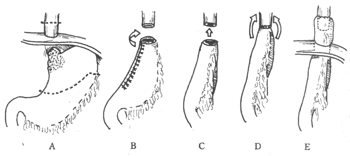
Figure 2: Partial resection of the esophagus and stomach, and end-to-end anastomosis of the esophagus and stomach for the treatment of cardiac cancer.
A. Resection range of the stomach and esophagus (between the dotted lines); B. The prepared gastric tube is rotated 90° clockwise; C. The gastric tube is pulled up and anastomosed with the esophagus; D. Completion of the inner layer full-thickness anastomosis; E. The gastric seromuscular layer is invaginated around the anastomosis for about 2.5 cm.
When tumor infiltration exceeds half the length of the lesser curvature of the stomach, total gastrectomy is required, which involves severing all five groups of gastric blood supply. After total gastrectomy, the duodenal end is sutured, and an esophagojejunostomy is performed. The simplest method is end-to-side esophagojejunostomy with side-to-side jejunojejunostomy, or Roux-Y esophagojejunostomy with end-to-side jejunojejunostomy. The author believes that the former method is simpler and preserves the jejunal blood supply better than the latter (Figure 3).
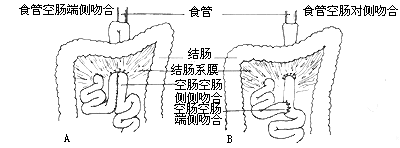
Figure 3: Gastrointestinal reconstruction after total gastrectomy for the treatment of cardiac cancer.
A. Total gastrectomy with end-to-side esophagojejunostomy and side-to-side jejunojejunostomy;
B. Total gastrectomy with Roux-Y esophagojejunostomy.
If the tumor has invaded the gastrosplenic ligament or the pancreatic tail, splenectomy and pancreatic tail resection can be performed simultaneously with subtotal or total gastrectomy. Care should be taken to properly suture the pancreatic cut surface, preferably covered with the greater omentum to prevent pancreatic fistula.
The extent of gastric resection in the surgical treatment of cardia cancer has always been a controversial issue. Some advocate for total gastrectomy in all cases, while others propose en bloc resection of the entire stomach, spleen, pancreatic tail, omentum, and regional lymph nodes to achieve improved survival. Some studies comparing subtotal and total gastrectomy found no difference in survival rates between the two, suggesting that total gastrectomy should only be performed when the tumor involves the gastric body. Other authors have found that prophylactic splenectomy during total gastrectomy does not benefit long-term survival in cases with splenic hilar lymph node metastasis, while in cases without splenic hilar lymph node metastasis, those who did not undergo splenectomy had higher survival rates. The splenectomy group also exhibited higher postoperative infection rates and faster recurrence-related deaths. In a report of 937 cases from the Cancer Hospital of the Chinese Academy of Medical Sciences, 10 patients underwent total gastrectomy, with 9 dying within one year and 1 not surviving beyond two years. Among 20 patients who underwent subtotal gastrectomy combined with splenectomy and pancreatic tail resection, 2 died postoperatively, and 2 survived for more than 5 years (1 for 6 years and 1 for 8 years). The authors agree with the view that since most cardia cancer cases are at an advanced stage at the time of diagnosis, with existing lymph node metastasis, radical surgery is of no benefit. If the tumor is indeed localized, radical surgery is unnecessary. For lesions confined to the cardia and not exceeding one-third of the lesser curvature length, subtotal gastrectomy with regional lymph node dissection is a more appropriate treatment strategy.
(III) Surgical Treatment and Short- and Long-term Outcomes of Cardia Cancer
The surgical outcomes for cardia cancer are worse than those for esophageal cancer. The resection rates in three major domestic groups range from 73.7% to 82.1%, with a resection mortality rate of 1.7% to 2.4%. The 5-year survival rates for these three groups are 19.0% to 24.0%, and the 10-year survival rates are 8.6% to 14.3%.
The main factors affecting the long-term survival of cardia cancer are the presence or absence of lymph node metastasis, whether the tumor has infiltrated the serosa membrane, and the nature of the resection (curative or palliative). The international TNM staging of cardia cancer, which incorporates the first two variable factors, is also an effective indicator for predicting patient outcomes.
(IV) Remnant Stomach Cardia Cancer
Reports of cancer occurring in the remnant stomach after distal partial gastrectomy are increasing. The incidence rate is 0.55% to 8.9%, with 16.4% to 58.5% of these cases occurring in the cardia region. The incidence of remnant stomach cardia cancer among all cardia cancers is 1.5% to 2.7%.
Definition of remnant stomach cancer:
1. The initial subtotal gastrectomy was performed for benign diseases, such as gastric or duodenal ulcers.
2. The interval between the initial partial gastrectomy and the occurrence of cancer is no less than 5 years. It is generally believed that it mostly occurs after Billroth II surgery, but there are also opposing views. Possible contributing factors include reduced gastric acid secretion after the initial gastrectomy, duodenal fluid reflux stimulation, and the presence of atrophic gastritis and intestinal metaplasia.
bubble_chart Metastasis and Spread
1. Direct infiltration and spread, involving the lower esophagus and other parts of the stomach, such as the hiatal region, diaphragm, left lobe of the liver, hepatogastric ligament, tail of the pancreas, splenic hilum, spleen, and other retroperitoneal structures.
2. Lymphatic metastasis, such as metastasis to the wall of the cardia, especially the submucosal and subserosal layers, which have rich lymphatic networks that communicate with the esophageal lymphatic network, converging to form extramural lymphatic vessels. These vessels drain upward to the mediastinum and downward to the celiac plexus, eventually entering the thoracic duct. Some authors propose three lymphatic drainage systems of the cardia: ① the ascending trunk, which runs upward along the esophageal wall to the mediastinum; ② the right trunk, which follows the lesser curvature of the stomach along the left gastric vessels and the cardia-esophageal branch to the celiac plexus; ③ the left trunk, which runs along the posterior wall and greater curvature to the upper edge of the pancreas and retroperitoneum. These can be further divided into the greater curvature branch, posterior gastric branch, and diaphragmatic branch. Lymph nodes are present along each system. The first-tier lymph nodes include the paracardial (left and right), lower esophageal, and lesser curvature lymph nodes. The second-tier lymph nodes include those along the left gastric vessels, splenic vessels, and omental lymph nodes. Distant lymph nodes include those along the celiac plexus, abdominal aorta, hepatic portal region, mediastinum, and supraclavicular lymph nodes.
3. Hematogenous metastasis: ① through the portal vein to the liver, then to the systemic circulation via the inferior vena cava; ② directly into the systemic circulation through inter-organ venous pathways. The former is the most common metastatic route.
4. Implantation: Cancer cells can detach and implant in the peritoneum, omentum, pelvic cavity, etc., potentially accompanied by bloody ascites.
The differential diagnosis of cardia cancer includes cardia spasm (achalasia), stenosis caused by chronic inflammation of the lower esophagus, and digestive ulcers at the cardia. The clinical characteristics of cardia spasm cases are young age, long medical history, and a long history of dysphagia, but they can still maintain a moderate health status. X-ray esophagography shows a symmetrical and smooth funnel-shaped stenosis above the cardia and significant dilation of the proximal esophagus.
Lower esophagitis is often accompanied by hiatal hernia and gastric reflux. Patients have a long history of heartburn and acid reflux, are often short and stout, and long-term inflammation leads to cicatricial stenosis, resulting in swallowing difficulties. X-ray barium meal shows stenosis of the lower esophagus and cardia, and the mucosa may be irregular. Esophagoscopy reveals inflammatory granulation and scar tissue, which can sometimes be difficult to distinguish from cancer by the naked eye. Repeated multiple biopsies with consistently negative results can confirm the diagnosis.




