| disease | Patent Ductus Arteriosus |
| alias | Patent Ductus Arteriosus |
The ductus arteriosus was originally a normal blood flow channel between the pulmonary artery and the aorta during the fetal period. At that time, the lungs did not perform respiratory functions, and the blood from the right ventricle flowed through the ductus into the descending aorta, while the blood from the left ventricle entered the ascending aorta. Therefore, the ductus arteriosus was essential for the special circulatory pattern during the embryonic period. After birth, the lungs expand and take on the function of gas exchange, with the pulmonary and systemic circulations each performing their respective roles. Soon after, the ductus closes on its own due to disuse. If it remains open, it constitutes a pathological condition known as patent ductus arteriosus (PDA), which requires surgical intervention to interrupt the blood flow. When patent ductus arteriosus coexists with cyanotic heart disease characterized by reduced pulmonary blood flow, the ductus becomes a vital condition for survival, and this situation should be considered separately. Patent ductus arteriosus is a relatively common congenital cardiovascular malformation, accounting for 12-15% of all congenital heart diseases. It is approximately twice as common in females as in males. About 10% of cases coexist with other cardiovascular malformations.
bubble_chart Pathological Changes
The ductus arteriosus is a conduit located between the base of the left pulmonary artery and the origin of the descending aorta. During the fetal period, the lungs are in a collapsed state, and the resistance of the pulmonary vasculature is high. The majority of blood ejected from the right ventricle into the pulmonary artery passes through the ductus arteriosus into the descending aorta. After birth, the lungs expand and contract with respiration, leading to a decrease in pulmonary vascular resistance. Consequently, blood ejected from the right ventricle enters both lungs for gas exchange. When the pressure in the pulmonary artery equals that in the aorta, the ductus arteriosus functionally closes. Subsequently, due to physiological disuse, changes in the angle of the ductus after lung expansion, and other yet unidentified factors, the ductus gradually undergoes histological closure, forming the ligamentum arteriosum. According to statistics, 88% of infants have closure of the ductus within two months after birth, and 98% have closure within eight months. If the ductus remains open by one year of age, the chance of spontaneous closure is low, resulting in patent ductus arteriosus (PDA).
The diameter and length of a patent ductus arteriosus typically range from a few millimeters to 2 cm. Occasionally, it may be as wide as the adjacent descending aorta and so short as to be almost negligible, representing a direct communication between the aortic and pulmonary arterial walls, known as a window-type patent ductus arteriosus.Patent ductus arteriosus results in a left-to-right shunt from the aorta to the pulmonary artery (Figure 6①). The volume of the shunt depends on the diameter of the ductus and the pressure gradient between the aorta and the pulmonary artery. Shortly after birth, the resistance and pressure in the pulmonary artery remain high, resulting in a small left-to-right shunt or only a systolic shunt. Subsequently, as the pulmonary artery resistance decreases and the pressure becomes significantly lower than that in the aorta, the shunt volume increases. Since the pulmonary artery receives both blood ejected from the right ventricle and the shunt flow through the ductus, the volume of blood returning to the left ventricle via the pulmonary veins increases, overloading the left ventricle and leading to left ventricular dilation, hypertrophy, and eventually heart failure. When the volume of blood flowing through the mitral valve orifice is excessive, relative mitral stenosis may occur. Impaired drainage and increased pressure in the pulmonary veins can lead to pulmonary interstitial edema. Increased blood flow through the ascending aorta and aortic arch causes dilation of these vessels; a similar response occurs in the pulmonary artery due to increased blood flow. Long-term increased pulmonary blood flow can induce reflex spasm of the small pulmonary arteries, and in the late stage (third stage), thickening and hardening of the small pulmonary arterial walls, narrowing of the lumen, and increased pulmonary vascular resistance may occur, exacerbating the pulmonary artery hypertension caused by increased pulmonary blood flow. This further burdens the right ventricle, resulting in combined left and right ventricular hypertrophy, and in advanced stages, right heart failure. As pulmonary vascular resistance increases and pulmonary hypertension progresses, the left-to-right shunt gradually decreases, eventually leading to a reverse (right-to-left) shunt, reduced oxygen saturation in the lower body, and cyanosis in the toes. Long-term turbulent flow can thin and weaken the ductus wall, leading to aneurysm formation or calcification. It also predisposes to infection, resulting in endarteritis. The proximal pulmonary artery may exhibit aneurysmal dilation due to increased intraluminal pressure.
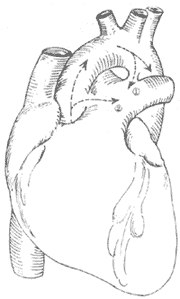
Figure 6: Schematic diagram of patent ductus arteriosus and aortopulmonary septal defect.
① Shows the ductus arteriosus between the descending aorta and the base of the left pulmonary artery; ② Shows the aortopulmonary septal defect between the ascending aorta and the main pulmonary artery.
bubble_chart Clinical Manifestations
The clinical manifestations of patent ductus arteriosus (PDA) mainly depend on the amount of blood shunted from the aorta to the pulmonary artery and whether secondary pulmonary hypertension and its severity occur. Mild cases may have no obvious symptoms, while severe cases may develop heart failure. Common symptoms include palpitations, shortness of breath, and fatigue after exertion, susceptibility to respiratory infections, and developmental delays. With the widespread use of antibiotics, bacterial endocarditis has become rare. In advanced stages, severe pulmonary hypertension can lead to reverse shunting, resulting in cyanosis of the lower body.
On physical examination, a typical sign is a loud continuous machinery-like murmur heard at the second intercostal space along the left sternal border, accompanied by a thrill. The second heart sound (P2) is accentuated but is often masked by the loud murmur. In cases with a large shunt, a diastolic murmur due to relative mitral stenosis may also be heard at the apex. Blood pressure measurements typically show normal systolic pressure with decreased diastolic pressure, resulting in a widened pulse pressure. Peripheral pulses may exhibit a water-hammer pulse and pistol-shot sounds.
In infants and young children, only a systolic murmur may be heard. In advanced stages with pulmonary hypertension, the murmur may vary significantly, presenting as only a systolic murmur or even disappearing, replaced by a diastolic murmur of pulmonary regurgitation (Graham Steell murmur).
Electrocardiogram (ECG) findings in mild cases may show no significant abnormalities. Typical ECG findings include left axis deviation, left ventricular high voltage, or left ventricular hypertrophy. In cases with significant pulmonary hypertension, both left and right ventricular hypertrophy may be present. In advanced stages, right ventricular hypertrophy predominates, with signs of myocardial damage.Echocardiography can visualize the patent ductus arteriosus and its communication between the aorta and pulmonary artery (Figure 7).
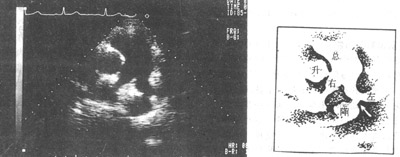
Figure 7: Echocardiographic image showing patent ductus arteriosus
↑ = Patent ductus arteriosus; Total = Main pulmonary artery
Left = Left pulmonary artery; Right = Right pulmonary artery
Ascending = Ascending aorta; Descending = Descending aorta
Chest X-ray findings include cardiomegaly, initially with left ventricular enlargement, and in advanced stages, right ventricular enlargement as well. In cases with a large shunt, the left atrium may also be enlarged. The ascending aorta and aortic arch shadows are widened. The pulmonary artery segment is prominent, and the pulmonary artery branches are dilated, with pulmonary vascular congestion (Figure 8). Sometimes, a "dancing hilum" sign may be seen on fluoroscopy.
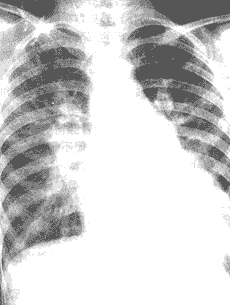
(1)
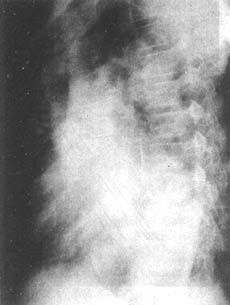
(2)
Figure 8: Chest X-ray of a patient with patent ductus arteriosus
(1) Posteroanterior view showing pulmonary vascular congestion, cardiomegaly with prominent left ventricular enlargement, widened aortic knob, and dilated main pulmonary artery.
(2) Left anterior oblique view showing significant left ventricular enlargement, with enlargement of the left atrium and right ventricle.
For cases where the diagnosis remains uncertain after the above examinations, right heart catheterization or retrograde aortography may be performed. The former may show pulmonary artery oxygen content exceeding that of the right ventricle by more than 0.5 volume percent, and pulmonary artery pressure and resistance can be measured. If the catheter passes through the ductus arteriosus into the descending aorta, the diagnosis is confirmed (Figure 9). Retrograde aortography can demonstrate contrast medium flowing through the ductus arteriosus into the pulmonary artery (Figure 10).
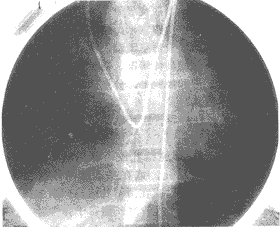
Figure 9: Patent ductus arteriosus
Right heart catheterization: The catheter is inserted from the right basilic vein, passing through the superior vena cava, right atrium, right ventricle, pulmonary artery, and ductus arteriosus, finally entering the descending aorta.
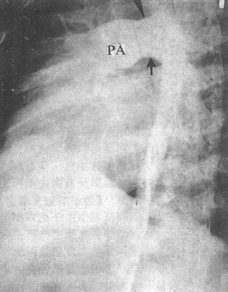
Figure 10
The femoral artery stirred pulse catheterization procedure. The image shows the contrast agent autonomously entering the pulmonary artery (PA) through a large stirred pulse catheter (↑).
bubble_chart Treatment Measures
After the diagnosis of patent ductus arteriosus (PDA) is confirmed, surgery should be performed at an appropriate time to interrupt the blood flow at the ductus, provided there are no contraindications (see below).
In recent years, for premature infants with respiratory distress syndrome caused by PDA, surgical treatment is also often recommended, while the use of drugs to promote ductal closure (prostaglandin synthase inhibitors - indomethacin) is less favored. This is because the dosage of the latter is difficult to control: a small dose may not be effective, while a large dose may cause side effects, or the ductus may reopen after stopping the medication.
Over the past decade, a few doctors in Germany, Japan, and other countries have pioneered a method involving combined venous and arterial catheterization. A Teflon sponge plug is introduced via a guidewire from the femoral artery into the PDA to occlude it (Figure 1). However, due to its limitation to PDAs with smaller lumens, frequent operational failures, or vascular injuries, this method has not been widely adopted.
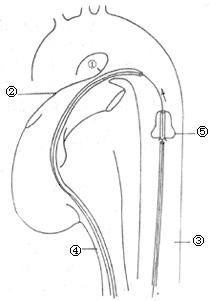
Figure 1: Arteriovenous catheterization for ductal occlusion
① PDA; ② Pulmonary artery; ③ Descending aorta; ④ Inferior vena cava; ⑤ Sponge plug
The closure of PDA is generally best performed before school age. If the shunt volume is large and symptoms are severe, surgery should be performed earlier. The risk of surgery increases and the efficacy decreases with older age and the development of pulmonary hypertension. In cases of bacterial endocarditis, surgery should be temporarily postponed, but if drug control of the infection is ineffective, surgery should still be pursued, with continued medication postoperatively, as the infection is often quickly controlled.
The following conditions are considered contraindications for surgery.
1. Concurrent cyanotic heart disease with reduced pulmonary blood flow, where the lesion causing cyanosis cannot be corrected simultaneously.
2. Cyanosis of the toes at rest or after grade I activity, or the presence of clubbing of the toes.
3. The murmur of PDA has disappeared, replaced by a diastolic murmur due to pulmonary hypertension causing pulmonary valve insufficiency (Graham Steell murmur).
4. Blood oxygen saturation at rest is below 95% or below 90% after activity, as measured by femoral artery blood oxygen.
5. Doppler ultrasound shows reverse (right-to-left) shunt at the ductus, or bidirectional shunt predominantly right-to-left.
6. Right heart catheterization shows total pulmonary resistance exceeding 10 Wood units.
[Surgical Methods and Techniques] The surgery generally uses a left posterolateral thoracotomy, entering the chest through the 4th intercostal space or by removing the 5th rib through the periosteum. The mediastinal pleura over the descending aorta is longitudinally incised centered on the ductus, and dissection is carried forward along the aortic surface until the ductus is exposed. This way, the left vagus nerve, recurrent laryngeal nerve, and the pericardial reflection at the pulmonary artery end of the ductus are pulled forward, away from the ductus itself, thus avoiding injury. A curved right-angle clamp (Mizusawa clamp) is slid along the aortic wall beneath the ductus to the posterior wall of the ductus. After the entire length of the ductus is freed, the method of ductal closure is chosen based on the specific conditions of the ductus, the available instruments, and the surgeon's skill and experience.
(1) Ductal ligation: This can be simple ligation or ligation with a pad.
1. Simple ligation: Two thick threads are passed around the ductus for double ligation, or a purse-string suture ligation is performed on the aortic side (Figure 2), or an additional transfixion suture ligation is added between the two ligation threads. This is suitable for long and elastic ducts.
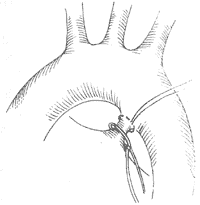
Figure 2: PDA ligation
A purse-string suture is made at the aortic end of the ductus, with the two threads not yet tightened.
2. Padded ligation involves using a polyester cloth strip as wide as the length of the duct, rolled into a cylindrical shape slightly narrower than the diameter of the duct. The free edge of the strip is sewn to the rolled body, and the thread from the middle section of the roll is reserved for later use. Both ends of the cloth roll are sewn to prevent loosening. The cloth roll is then placed along the duct, and two thick threads are wrapped around the duct to ligate it, with each ligation thread tied to the reserved thread on the cloth roll to prevent the padded roll from slipping (Figure 3). This method applies force through the ligation threads on the padded roll to compress and close the duct lumen, while the pulling force of the ligation threads on the duct wall is minimal, avoiding the risk of the duct wall being torn by the ligation threads and the possibility of duct recanalization, as seen in simple ligation. Padded ligation is particularly suitable for cases where the duct is large and the duct wall has poor elasticity (such as in cases complicated by pulmonary stirred pulse hypertension or previous ductal membrane inflammation).
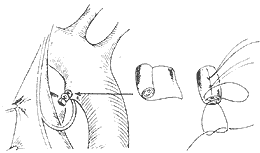
Figure 3 Overview of the ligation of the stirred pulse duct with padding. The right side shows a schematic diagram of the padding roll.
(2) Duct transection and suturing: Use two specialized non-traumatic duct clamps to clamp the main stirred pulse side and the pulmonary stirred pulse side of the duct respectively. If the duct is short, the main stirred pulse end can be clamped on the descending aorta using a long curved stirred pulse clamp or Potts-Smith clamp to extend the length of the duct. Between the two clamps, continuously suture the main stirred pulse cut end of the duct with 3-0 non-traumatic needle thread while cutting. After the duct is cut, continue to suture back to the starting point and tie a knot, then continuously suture the pulmonary stirred pulse cut end of the duct (Figure 4). Duct transection and suturing require reliable quality duct clamps and good vascular suturing techniques, otherwise there is a risk of fatal bleeding during surgery.
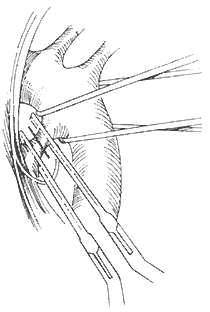
Figure 4 Stirred pulse duct transection and suturing
Two reflective duct clamps are clamped at both ends of the duct, the dotted line indicates the intended cut. The proximal and distal main stirred pulse of the duct are wrapped with gauze to control bleeding in case of emergency. After the duct is cut, the two cut ends are sutured closed.
(3) Duct clamping: Suitable for ducts with a diameter of less than 2cm and good wall elasticity. Use a specially made stirred pulse duct clamp to clamp the main stirred pulse end and the pulmonary stirred pulse end once each, so that the rows of titanium nails inside the clamp pass through the front and back walls of the duct and bend to press it closed (stapler principle) (Figure 5). Due to the small local operating space, it is sometimes difficult to properly place the clamp, which may even lead to traumatic hemorrhage of the duct wall, so caution is required.
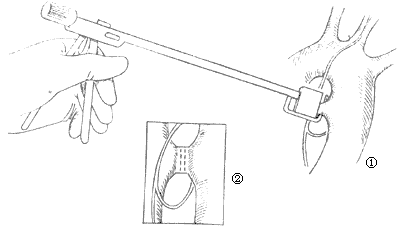
Figure 5 Stirred pulse duct clamping
① The clamp is already placed on the duct ② The duct is clamped (two rows of titanium nails can be seen)
(4) Other surgical methods: For cases of duct re-canalization after surgery, the pericardium can be cut open and a thick thread with padding can be tied around the pulmonary stirred pulse origin of the duct. For cases of duct wall calcification, stirred pulse aneurysm formation, or window-shaped stirred pulse duct, the main stirred pulse can be cut open under the condition of blocking the proximal and distal main stirred pulse blood flow, and the main stirred pulse internal opening of the duct can be sutured with a Dacron patch. To prevent spinal ischemic injury caused by blocking the main stirred pulse blood flow, it is advisable to perform under hypothermic anesthesia, and if necessary, use the proximal and distal main stirred pulse bypass method - "bridge" between the proximal and distal ends of the blocked main stirred pulse with a specially made pipe with a diameter of not less than 1cm and anti-coagulation inner wall, so that when the main stirred pulse is blocked, blood can supply the descending aorta through the pipe; if there is no specially made pipe, a high-quality plastic pipe can also be used, but to prevent blood coagulation in the plastic pipe during the bypass process, heparin can be injected intravenously at 1mg/kg. Recently, for those who cannot safely complete the surgery with the above methods, a median sternotomy is used, under hypothermic conditions of extracorporeal circulation, the pulmonary stirred pulse is cut open, and the duct opening is sutured closed or patched with a Dacron patch from the inside; to prevent middle qi from entering the main stirred pulse during the operation, a head-down position should be taken, and a low-flow perfusion method should be used, so that systemic stirred pulse air embolism can be prevented without affecting the operation due to excessive blood flow at the duct opening.
For cases where pulmonary stirred pulse hypertension has reached a critical level, a duct blocking test should be performed before closing the duct during surgery. If there is no significant change in blood pressure and electrocardiogram within the 15-minute blocking period, the duct closure surgery can be completed, otherwise the surgery should be abandoned.
To enhance the safety of the stirred pulse catheter closure procedure, gauze bands can be placed around the proximal and distal ends of the main stirred pulse near the catheter before dissecting and handling the catheter, so that the gauze bands can be tightened to control bleeding in case of an emergency. Before performing the catheter closure, the anesthesiologist should administer appropriate hypotensive measures to maintain the systolic pressure of the stirred pulse at around 12.0 kPa (90 mmHg), which helps reduce the risk of catheter rupture and bleeding.
The surgical mortality rate due to massive bleeding during stirred pulse duct closure surgery depends on factors such as the texture of the duct wall, the surgical method used for duct closure, and the skill level of the surgeon, and should generally be within 1%. Simple ligation or clamping of the duct may result in postoperative recanalization, with a recanalization rate generally above 1%. The recanalization rate after padded ligation is lower than the former two. The long-term effectiveness of stirred pulse duct closure surgery depends on whether there were secondary sexually transmitted disease changes in the pulmonary vessels before surgery and their extent. Patients who undergo surgery before the onset of pulmonary vascular disease can fully recover and have a normal lifespan; those with severe and irreversible pulmonary vascular disease will still have high pulmonary vascular resistance and heavy right heart load postoperatively, resulting in poorer outcomes.
Patent ductus arteriosus should be differentiated from cases with similar heart murmurs, such as aortopulmonary septal defect, aortic sinus rupture into the right ventricle or right atrium, coronary-right heart chamber or pulmonary fistula, and high ventricular septal defect with aortic valve prolapse (insufficiency). The diagnosis can generally be made based on the location of the murmur and ultrasound imaging. If necessary, right heart catheterization or (and) cardiac angiography should be performed for confirmation.




