| disease | Malignant Tumor of the Large Intestine |
| alias | Malignant Tumor of Colon |
Malignant tumors of the large intestine originate from the mucosal epithelium or submucosal mesenchymal tissue. Among them, malignant tumors arising from the mucosal epithelium are collectively referred to as colorectal cancer, which is the most common malignant tumor in the gastrointestinal tract. Those originating from mesenchymal tissue are called sarcomas, accounting for about 1% of malignant tumors in the large intestine, with leiomyosarcoma being the most common. Rare types include smooth muscle sarcoma, fibrosarcoma, and angiosarcoma.
bubble_chart Pathogenesis
Colon cancer refers to the malignant transformation of the epithelial cells of the colon mucosa under the influence of various carcinogenic factors such as environmental or genetic factors. It has a poor prognosis and high mortality rate, making it one of the common malignant tumors in China. Clinically, it is more prevalent in adults over 40 years old, with early symptoms being insidious. In advanced stages, symptoms such as changes in bowel habits, hematochezia, weight loss, abdominal pain, and abdominal masses may appear, and even intestinal obstruction may occur. Diagnosis often requires colonoscopy or barium enema X-ray examination. Once diagnosed, a comprehensive treatment approach primarily involving surgery is recommended. Due to the poor prognosis of mid-to-advanced stage colon cancer, current efforts advocate for screening asymptomatic high-risk populations to detect early-stage colon cancer. Actively researching the causative factors of colon cancer and implementing preventive measures against these factors may be of significant importance in reducing the incidence of colon cancer.
Colon cancer is one of the common malignant tumors that seriously threaten human life and health. Epidemiological data worldwide indicate that among various malignant tumors, colon cancer ranks third in incidence, with an annual increase of up to 570,000 new cases, accounting for 9% of all diagnosed cases. In China, the lower reaches of the Yangtze River and the southeastern coastal regions, including Jiangsu, Shanghai, Zhejiang, Fujian, Taiwan, and Hong Kong, are high-incidence areas. In some high-incidence regions, such as Jiashan County in Zhejiang Province, the annual incidence rate is as high as 22.4 per 100,000 people, and the annual mortality rate is 18.38 per 100,000 people, ranking first among malignant tumors. With the development of the social economy, the expansion of modern production scales, the improvement of living standards, and the resulting ecological and physiological negative effects, the incidence of colon cancer is expected to show an annual increasing trend. Some predictions based on the increasing incidence of various tumors over the years suggest that the standardized incidence rate of intestinal cancer will rise by 66% between 1980 and 2000. A similar trend is observed domestically. Some data indicate that in the 1960s, the incidence of colon cancer ranked sixth among malignant tumors, but it has now more than doubled, jumping to fourth place and ranking second among malignant tumors in women.Colon cancer can occur in any segment of the colon, but it is more common in the left colon, particularly the rectum and sigmoid colon. From 1987 to the present, Southern Hospital has detected 386 cases of colon cancer through endoscopy (including 78 cases of adenoma malignant transformation and 2 cases of juvenile polyp malignant transformation). Among these, the rectum accounted for 58.6%, the sigmoid colon for 21.2%, the descending colon for 1.8%, the transverse colon (including the hepatic and splenic flexures) for 8.0%, and the cecum and ascending colon for 10.4%. This distribution is largely consistent with the 611 adenomas removed and biopsied via endoscopy. The reason why colon cancer is more prevalent in the distal colon is not entirely clear. Some authors attribute this to the absorption of water in the intestinal contents in this segment, leading to excessively high concentrations of carcinogens.
Most colon cancers are single lesions, but about 5% are multiple. Multiple cancers are classified as synchronous or metachronous. The former refers to the presence of two or more cancerous lesions in the colon of the same patient simultaneously, while the latter refers to the sequential appearance of cancerous lesions in the same patient. These findings suggest that during endoscopic examinations, in addition to carefully checking other intestinal segments for cancerous lesions in patients with a single cancer, postoperative follow-up should also be emphasized to detect residual synchronous or metachronous multiple cancers. In our past experience, among 275 cases, we found 9 cases of multiple cancers, accounting for 3.3%. Of these, 5 were clearly synchronous, 1 was metachronous, and 3 were of undetermined timing.The occurrence of large intestine cancer is the result of multiple genetic alterations in the colonic mucosal epithelium caused by the combined effects of genetic and environmental factors. The earliest change is the dysregulation of crypt cell growth, leading to a hyperproliferative state. As cells proliferate and accumulate, the crypt proliferative zone becomes fragmented and shifts upward, gradually forming a mass (polyp) that protrudes into the intestinal lumen. When tumor cells infiltrate the basement membrane, it can be considered cancerous, and further progression may lead to metastasis. The above dynamic process is illustrated in Figure 1.
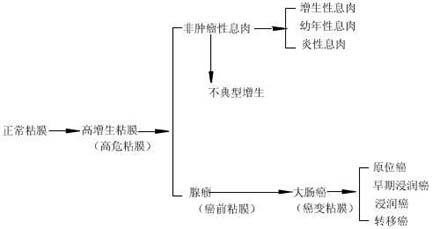
Figure 1 Schematic diagram of the development and metastasis dynamics of large intestine cancer
In the above-mentioned process of carcinogenesis, a state of high proliferation is a fundamental prerequisite for the cancerous transformation of the large intestine mucosa, hence it is referred to as high-risk mucosa. Its progression can lead to the formation of polyps. Since adenomatous polyps exhibit atypical hyperplasia and are closely related to large intestine cancer, they are also termed precancerous mucosa. If appropriate intervention, such as the removal of adenomas, is administered at this stage, the development of carcinogenesis can be halted, thereby reducing the incidence of large intestine cancer. Non-neoplastic polyps can also, in rare cases, progress to cancer through atypical hyperplasia. For example, we have previously reported cases of juvenile polyp carcinogenesis.
1. Normal MucosaThe colonic mucosa is primarily composed of mucosal epithelium, with the epithelium extending from the surface into the deeper layers to form long, straight tubular glands that occupy the entire thickness of the mucosa. These glands are collectively called Lieberkühn crypts and mainly consist of glandular epithelial cells. The epithelial cells at the base of the crypts are highly proliferative and serve as the stem cells of the intestinal mucosa. These stem cells have the potential to generate goblet cells and columnar cells, maintaining the normal renewal of intestinal mucosal cells. When dead intestinal epithelial cells shed into the intestinal lumen, the basal cells of the crypts proliferate and migrate toward the mucosal surface, gradually maturing and replacing the dead epithelial cells.
The proliferative zone of normal colonic crypts is confined to the lower one-third of the crypts. Under normal conditions, it takes approximately 3 to 8 days for intestinal epithelial crypts to differentiate into functional goblet cells or absorptive cells and migrate to the crypt surface. This process is regulated by various factors, including mechanical stimulation from intestinal contents, neural and endocrine hormone regulation, as well as the influence of digestive products and gut microbiota. In addition to epithelial cells, crypts also contain other types of cells. One type is the enteroendocrine cells, primarily located at the base of the crypts, with projections that communicate with neighboring epithelial cells. These cells may function as paracrine cells, affecting the biological behavior of adjacent cells through the local release of peptide products. The second type is the pericryptal fibroblasts, which envelop the entire crypt from the base to the mucosal surface and form the lamina propria, providing structural support for the crypts. This stromal-epithelial interaction may also play a significant role in regulating crypt cell maturation.
2. High-Risk Mucosa
The characteristic change of high-risk mucosa is the expansion of the crypt proliferative zone. The factors causing dysregulation of crypt proliferation are not yet fully understood. In addition to inherent genetic factors, environmental factors, particularly dietary factors, may play a crucial role in influencing the regulation of crypt cell proliferation. High-fat diets, excessive consumption of pickled or grilled foods, and salted fish can promote the proliferation of intestinal mucosal epithelium. This is because high-fat diets increase the levels of bile acids and neutral cholesterol in the intestinal lumen while altering the colonic microbiota, leading to an increase in anaerobic bacteria. These bacteria release acids and degrade neutral cholesterol, synthesizing or converting them into carcinogens, thereby disrupting crypt cell proliferation. Recently, researchers have linked intraluminal cell proliferation stimulation with free radical mechanisms, proposing that during digestion, food releases iron, which binds with bile pigments or amino acids, increasing fecal iron content. In the predominantly anaerobic environment of the colon, most iron is reduced to ferrous form. Aerobic and microaerophilic microorganisms on the relatively oxygen-rich surface of fecal matter produce superoxide ions and peroxides, which further generate hydroxyl radicals via the Fenton reaction. These radicals initiate oxidative chain reactions and convert unsaturated lipids and dietary precarcinogens into carcinogens and tumor promoters. This hypothesis has been partially supported by research findings, such as the isolation of highly free radical-active substances from the feces of large intestine cancer patients, which have been shown to strongly promote the proliferative activity of intestinal epithelial cells.
Through the study of cell dynamics, abnormalities in the proliferation mechanics of high-risk mucosal cells can be detected. The classical method involves short-term in vitro incubation of mucosal tissue biopsies obtained via endoscopy for tritiated thymidine incorporation assays. In normal mucosa, labeled cells are located in the lower two-thirds of the crypts, whereas high-risk mucosa exhibits an increase in labeled cells. Recently, the use of monoclonal antibodies that specifically recognize cell cycle phases for labeling proliferating cells, or the application of cell microspectrophotometry and flow cytometry for DNA content measurement, has greatly simplified observation methods. We have comparatively observed changes in proliferating cell nuclear antigen and rRNA gene activity, as well as analyzed cellular DNA content, all of which revealed progressive alterations in crypt proliferating cells during the process of carcinogenesis. Additionally, certain enzyme markers related to cell proliferation have garnered significant attention, such as ornithine decarboxylase, which shows marked differences even in the apparently normal intestinal mucosa of patients with familial polyposis compared to healthy individuals.
III. Precancerous mucous membrane
Precancerous mucous membrane is characterized by atypical hyperplasia. All adenomas exhibit atypical hyperplasia; therefore, adenomatous polyps are a recognized precancerous lesion. Among the 1,973 cases of large intestine polyp biopsies we completed, adenomas accounted for 1,048 cases (53.1%), with an adenoma carcinogenesis rate of 7.4%. In-depth studies have shown that the adenoma carcinogenesis rate is closely related to the amount of villous components, the degree of atypical hyperplasia, as well as the size and number of adenomas. For example, the carcinogenesis rate of villous adenomas is 39.3%, while that of tubular adenomas is only 0.9%.
The development of large intestine cancer is a multistage progressive process. Researchers represented by Morson proposed the adenoma-carcinoma sequence theory as early as the 1960s, suggesting that the occurrence of large intestine cancer originates from the malignant transformation of adenomas. The natural progression of adenoma development, the consistency between high-risk populations, ethnic groups, and age groups for large intestine cancer and adenoma occurrence, as well as the alignment between the frequency of intestinal cancer segments and the distribution of adenomas, all support this view. For example, Prager et al. followed up with 283 patients with adenomatous polyps for at least 15 years, finding that 4.3% developed cancer. We followed up with 287 adenoma cases, but the interval was too short to reflect the natural course of adenomas. Even so, one case of carcinogenesis was identified. It is generally believed that adenomas take about 10 years to develop—approximately 5 years from normal mucous membrane to adenoma formation and another 5 years from adenoma to cancer.
Histological observations can reveal the intrinsic connection between atypical hyperplasia and carcinogenesis. Transitional or concurrent atypical hyperplastic glands are often observed at the edges of cancerous lesions, and atypical hyperplasia is a fundamental feature of adenomas. Additionally, other precancerous lesions such as ulcerative colitis and schistosomiasis are also closely associated with atypical hyperplasia of the mucous membrane epithelium when they develop into cancer. These results can be replicated in experimental animals using chemical carcinogenesis methods, particularly in mice. In our study of dimethylhydrazine-induced large intestine cancer in rats, although adenomatous polyps were often absent before cancer formation, histological, autoradiographic, DNA quantitative analysis, and ultrastructural studies confirmed that the majority of cancers originated from atypical hyperplastic lesions of the mucous membrane epithelium, consistent with the adenoma-carcinoma sequence.
However, current data indicate that clinically, some small early cancers show no residual adenoma or atypical hyperplasia. Therefore, some authors suggest that they arise directly from stem cells in the germinal center of the normal large intestine mucous membrane, unrelated to large intestine polyps. Such cancers are termed De Novo cancers, first proposed by Castleman et al. The proportion of these cancers is difficult to assess because even if adenoma remnants are rarely found in some large intestine cancer tissues, it does not necessarily confirm their origin as De Novo cancers. This is because cancer cells can destroy adenoma cells themselves. For example, Friedman et al. found in in vitro culture studies that when cancer cells were co-incubated with adenoma cells, the former exhibited invasive and destructive effects on the latter.
IV. Carcinomatous mucous membrane
On the basis of atypical hyperplasia, further enlargement of glandular epithelial cell nuclei, abnormal nuclear-to-cytoplasmic ratio, and pathological mitosis can be considered as cancerous transformation. However, clinically, when the cancerous glands have not infiltrated beneath the mucous membrane (i.e., carcinoma in situ), good therapeutic outcomes can be achieved through simple endoscopic removal. Therefore, to avoid unnecessary complex treatment, it is generally classified only as grade III atypical hyperplasia. The cancerous mucous membrane exhibits genetic instability, and the progression of grade III atypical hyperplasia to invasive cancer is merely one stage in the development of the cancerous mucous membrane, with inevitable progression leading to cancer metastasis. It is generally believed that a 1cm diameter large intestine polyp-like cancer takes about 2 years to develop into a circumferential lesion that invades the entire intestinal wall. Early intervention in this developmental process of large intestine cancer can yield varying clinical outcomes. For instance, carcinoma in situ can be completely cured after resection, early invasive cancer can achieve excellent therapeutic results, but the 5-year survival rate after surgical resection of metastatic cancer is less than 40%. Therefore, early detection and diagnosis of large intestine cancer are currently the focal points of large intestine cancer prevention and treatment research.
Molecular biological studies on colonic tissues at different stages of large intestine mucosal carcinogenesis have revealed that each phase of large intestine carcinogenesis has a molecular biological basis. There are primarily four types of genetic-level changes: point mutations in the ras gene and partial deletions of chromosomes 5, 17, and 18. These non-random genetic alterations appear to occur sequentially, with the earliest change likely being point mutations in the ras oncogene, observed in 70% of adenomas and 52% of cases with high-grade dysplasia, but only in 9% and 7% of small adenomas and grade I dysplasia, respectively. Activation of the ras gene can induce transformation in certain tumor models, and this activation may play a significant role in regulating cell growth. It is hypothesized that ras gene mutations may facilitate the transformation of colon cells into adenomas, though they may not necessarily lead to carcinogenesis on their own. Deletions in chromosomes 17 and 18 are found in 75% of adenocarcinomas, 47% of carcinomatous adenomas, and 6% of small adenomas. These regions are believed to harbor tumor suppressor gene loci, and the loss of such genes may contribute to cancer progression. Recently, we investigated the expression of oncogene products such as myc, ras, c-rebB-2, and P53 during large intestine carcinogenesis. Synchronous detection analysis showed that the simultaneous positive expression of two or more oncogene products occurred in 19.4% of adenomas and 52.9% of adenocarcinomas. Further analysis indicated that oncogene expression in adenomatous lesions correlates with the degree of dysplasia. For instance, dual or more oncogene expression was observed in only 8.3% of grade I dysplasia, 33.3% of grade II, and 55.6% of grade III. In contrast, oncogene expression in large intestine cancer showed no clear relationship with the degree of tumor differentiation, suggesting that multigene alterations may occur during large intestine carcinogenesis, though these changes may not necessarily be linked to cancer progression.
bubble_chart Pathological Changes
1. Basic Morphological Features of Large Intestine Cancer
Grossly, large intestine cancer typically presents as mass-like, ulcerative, or infiltrative appearances, though early-stage cancers may not exhibit these characteristics.
1. Early-Stage Cancer Early-stage large intestine cancer refers to cases where cancer infiltration is limited to the mucosa and submucosa. If infiltration reaches the muscular layer, it is termed advanced cancer. According to Maruyama's classification under endoscopy, early-stage large intestine cancer is categorized as follows: - **Type I (Polypoid Elevated Type)**: Further divided into pedunculated (Ip), semi-pedunculated, or sessile (Is). This type is often intramucosal carcinoma, with a diameter <2.0 cm, appearing red and uneven on the surface, sometimes with erosion, making it difficult to distinguish from benign polyps. - **Type IIa (Flat Elevated Type)**: Resembles a coin in shape. If accompanied by an ulcer, it is termed **IIa + IIc**, presenting as a small dish-like lesion with raised edges and a central depression. These types are more common in submucosal cancers, usually larger (>2.0 cm), with surface erosion or exudate. Notably, the diagnosis and classification of early-stage large intestine cancer rely on the depth of cancer cell infiltration. Thus, endoscopic diagnosis must be confirmed by histopathological examination of surgically resected bowel segments or endoscopically removed polyps. Endoscopy and biopsy alone are insufficient for definitive early-stage diagnosis.
2. Advanced Cancer Manifests as elevated (polypoid), ulcerative, infiltrative, or colloid types.
(1) **Polypoid Cancer (Borrmann Type I)**: The tumor primarily grows into the intestinal lumen, appearing as broad-based polyps ranging from 1–2 cm to 10 cm. The surface is uneven, often cauliflower-like with scattered erosions and small ulcers, prone to bleeding. More common in the right colon due to its larger lumen and thinner contents, facilitating intraluminal growth.
(2) **Ulcerative Cancer**: Further divided into localized and infiltrative subtypes: - **Localized Ulcerative Cancer (Borrmann Type II)**: Endoscopically, the tumor has clear margins with a large ulcer and nodular raised edges resembling a crater. - **Infiltrative Ulcerative Cancer (Borrmann Type III)**: Tumor margins are indistinct due to wall infiltration. The surface shows congestion, edema, and scattered erosions/ulcers, bleeding easily on contact. Progression may lead to circumferential narrowing. Most common type, seen in both left and right colon, though deep ulcers are rare in the latter.
(3) **Infiltrative Cancer (Diffuse Infiltrative, Borrmann Type IV)**: Cancer cells diffusely infiltrate the bowel wall, thickening it. Mucosal nodules are rare, but scattered erosions/small ulcers are seen. Connective tissue hyperplasia often hardens the lesion, causing tubular stenosis (also called **sclerotic cancer**). Predominantly occurs in the left colon, especially the rectum and sigmoid colon.
(4) **Colloid Cancer (Borrmann Type V)**: A special variant with variable morphology, often presenting as a mass with villous/papillary projections. Endoscopically, abundant gelatinous mucus is observed, soft and elastic, with indistinct borders. More common in the ascending colon and cecum, but also seen in the rectum.
3. **Recurrent Cancer at Anastomotic Site**: Confirmed by histopathological biopsy. Endoscopically, it appears as an elevated mass at the anastomosis with congestion, edema, erosion, bleeding, and purulent exudate, often causing stenosis.
II. **Histological Types and Clinicopathological Staging of Large Intestine Cancer**
1. Histological classification of large intestine cancer Histologically, it can be divided into papillary adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma, signet-ring cell carcinoma, undifferentiated carcinoma, adenosquamous carcinoma, and squamous cell carcinoma, among other types. Among these, adenocarcinoma is the most common, accounting for 93-95%, while mucinous carcinoma accounts for 4.9-6.6%.
2. Clinical Pathological Staging The depth of cancer infiltration does not penetrate the muscular layer, and there is no lymph node metastasis.
A1 Stage The cancer infiltration is limited to the mucosal layer (M cancer) and submucosal layer (Sm cancer) of the large intestine.
A2 Stage The cancer has invaded the superficial muscular layer (Pm cancer).
A3 Stage The cancer has invaded the deep muscular layer.
Dukes B Stage The tumor has penetrated the muscular layer and may invade the serosa, beyond the serosa, or the surrounding rectum, but there is no lymph node metastasis.
Dukes C Stage The tumor is accompanied by lymph node metastasis.
Dukes D Stage The tumor is accompanied by distant organ metastasis.
The histological type and progression degree of large intestine cancer have a certain impact on prognosis. In terms of histological type, the prognosis of adenocarcinoma is better than that of mucinous adenocarcinoma, signet ring cell carcinoma, and undifferentiated carcinoma. Among adenocarcinomas, well-differentiated types have a better prognosis than poorly differentiated ones. However, the degree of disease progression better reflects the prognosis of large intestine cancer. For example, Jarvinen et al.'s follow-up study of 249 surgical patients with large intestine cancer showed that the 5-year survival rates for Dukes A, B, C, and D stages were 88%, 61%, 26%, and 1%, respectively. Turunen et al.'s data also showed similar results, with rates of 82%, 54%, 22%, and 2%, respectively.
bubble_chart Clinical Manifestations
Early-stage large intestine cancer symptoms are not obvious and may be asymptomatic or only present with vague discomfort, indigestion, occult blood, etc. As the cancer progresses, symptoms gradually become more apparent, manifesting as changes in bowel habits, hematochezia, abdominal pain, abdominal masses, intestinal obstruction, as well as systemic toxic symptoms such as fever, anemia, and weight loss. Tumor infiltration and metastasis can also cause changes in corresponding organs. Large intestine cancer presents different clinical signs and symptoms depending on its primary site.
I. Right-sided intestinal cancer
The prominent symptoms are abdominal masses, abdominal pain, and anemia. Some patients may experience mucus or bloody mucus stools, frequent bowel movements, abdominal distension and fullness, and intestinal obstruction, though these are far less common than in left-sided colon cancer. The right colon has a wider lumen, and primary cancers are often already large when discovered, mostly presenting as ulcerated masses. Many patients may palpate a mass in the right abdomen. Unless the cancer directly involves the ileocecal valve, intestinal obstruction is relatively rare. Since stool in the right colon remains semi-fluid and mushy, bleeding caused by stool friction against the tumor is less common. Most bleeding results from cancerous necrosis and ulceration, and because the blood mixes uniformly with stool, it often goes unnoticed, leading to long-term chronic blood loss. Patients frequently seek medical attention due to anemia. Abdominal pain is also common, usually a dull ache caused by tumor invasion of the intestinal wall. Secondary infection of cancerous ulcers can lead to local tenderness and systemic toxemia.
II. Left-sided intestinal cancer
The prominent symptoms are changes in bowel habits, bloody mucus stools or hematochezia, and intestinal obstruction. The left colon has a narrower lumen, and primary cancers often grow in a ring-like infiltrative pattern, easily causing narrowing of the intestinal lumen, so constipation is common. Subsequently, as fluid accumulates in the lumen above the narrowing and intestinal peristalsis increases, diarrhea may follow constipation, often alternating between the two. Since stool becomes more solid as it enters the left colon, visible hematochezia caused by stool friction against the tumor is more common. Patients often seek medical attention earlier, and anemia due to long-term chronic blood loss is less prominent than in right-sided colon cancer. Tumor infiltration into the intestinal wall causing narrowing often leads to chronic incomplete intestinal obstruction, with patients experiencing prolonged difficulty in bowel movements and intermittent abdominal pain. Due to the lower location of the obstruction, vomiting is usually not prominent.
III. Rectal intestinal cancer
The prominent symptoms are hematochezia, changes in bowel habits, and associated symptoms caused by advanced-stage tumor infiltration. The primary cancer is often located lower, and the stool is harder, making the tumor more prone to friction-induced bleeding, which is usually bright or dark red and either separate from formed stool or attached to its surface, leading to misdiagnosis as "hemorrhoidal" bleeding. Due to tumor stimulation and secondary infection of ulcerated masses, persistent defecation reflexes may occur, often misdiagnosed as "bacillary dysentery" or "enteritis." Ring-like tumor growth causes narrowing of the intestinal lumen, initially presenting as deformed or thinned stool and later as incomplete obstruction in advanced stages.
IV. Tumor infiltration and metastasis
Local extension is the most common form of infiltration in large intestine cancer. Tumor invasion of surrounding tissues often causes corresponding symptoms, such as rectal cancer invading the sacral nerve plexus leading to persistent lower abdominal and lumbosacral pain and fecal incontinence. Due to the shedding of cancer cells, rectal examination may reveal masses in the bladder-rectal or uterus-rectal fossa. Widespread dissemination can lead to ascites. Early-stage cancer may also spread along the lymphatic spaces around the intestinal wall nerves, later metastasizing to lymph nodes via lymphatic vessels. When cancer cells metastasize to the para-aortic lymph nodes and enter the cisterna chyli, they can spread via the thoracic duct to the left supraclavicular lymph nodes, causing swelling there. A few patients may experience retrograde dissemination due to obstruction of the ascending lymphatic vessels by tumor emboli, leading to numerous diffuse nodules in the perineum. In female patients, tumors may metastasize to both ovaries, causing Krukenberg's tumor. Advanced-stage large intestine cancer can also metastasize hematogenously to the liver, lungs, bones, and other sites.
bubble_chart Auxiliary Examination
1. Laboratory Tests
In addition to performing a complete blood count to assess whether the patient has anemia, other laboratory tests can be conducted based on diagnostic and differential diagnostic needs. Among these, the fecal occult blood test and the detection of biological markers for large intestine cancer hold significant value for the early diagnosis of large intestine cancer.
1. Fecal Occult Blood Test Since large intestine cancer often presents with varying degrees of bleeding due to mucosal erosion and ulcers, the simple and convenient fecal occult blood test can be used to monitor large intestine cancer. Early fecal occult blood tests relied on chemical colorimetric methods, commonly using reagents such as benzidine or guaiac. In recent years, these have gradually been replaced by more specific immunochemical occult blood reagents. However, since the fecal occult blood test cannot distinguish between cancerous and non-cancerous bleeding, it is currently primarily used as an initial screening tool for large-scale population-based large intestine cancer screening. Nonetheless, a small number of early cancers may yield false-negative results, leading to misdiagnosis.
2. Rectal Mucosal T-Antigen Test Also known as the galactose oxidase test, this method detects specific markers for large intestine cancer and precancerous lesions. Jianbian Fang By simply smearing mucus collected from a rectal examination onto specially prepared paper membranes or slides, the presence of T-antigen expression in the patient's intestinal mucosa can be determined through the galactose oxidase reaction and Schiff's reagent staining. Clinical and screening validations have shown that this method has high sensitivity and specificity for detecting large intestine cancer. When used in screening, it complements the immunochemical occult blood test for large intestine cancer, though it still carries certain rates of false positives and false negatives.
3. Serum CEA Testing Most large intestine cancer patients exhibit elevated serum CEA levels, often exceeding 50 μg/ml. However, this test lacks strong specificity, as serum levels can also rise in some non-digestive tract tumors and benign conditions. Additionally, CEA has poor sensitivity for early-stage intestinal cancer and adenomatous polyps, making its effectiveness for early large intestine cancer detection limited. In 1982, Magagi et al. developed CA19-9 by immunizing mice with human intestinal cancer cell lines, which can recognize highly cancer-specific sialylated gangliosides. Their findings showed elevated levels in 19–49% of colorectal tumors. However, CA19-9 is more sensitive for gastric, pancreatic, hepatic, and biliary tract cancers and is not more sensitive than CEA for serological detection of large intestine cancer.
Other tests, such as the detection of large intestine cancer-associated antigens, ornithine decarboxylase, serum sialic acid levels, and leukocyte adhesion inhibition tests, have shown some efficacy in studies. However, their specificity and sensitivity for clinical application still require further improvement.
2. Endoscopic Examination
This has become widely used in clinical practice and is often preferred over conventional X-ray examinations by experienced endoscopists. For highly suspicious clinical cases of large intestine cancer, a full colonoscopy is particularly recommended to avoid misdiagnosis. Due to its safety and reliability, fiber-optic colonoscopy not only allows for the assessment of tumor size, morphology, location, and mobility but also enables the removal of polyps or early microcarcinomas. Suspicious lesions can be biopsied under direct visualization, making it the most effective diagnostic tool for large intestine cancer today. In large intestine cancer screening, it is often regarded as the "gold standard" for evaluating the effectiveness of various initial screening methods.
3. X-ray Examination
This can detect lesions that are not visible through proctoscopy or sigmoidoscopy and is particularly useful for patients in whom fiber-optic colonoscopy cannot reach the ileocecal region. It is also an effective diagnostic tool for large intestine cancer. Barium enema examinations are commonly performed, with key findings including local mucosal deformation, abnormal peristalsis, intestinal lumen narrowing, and filling defects. However, detecting smaller lesions, especially early-stage cancers with diameters less than 2 cm, can be challenging. The use of double-contrast barium enema (air-barium contrast) can aid in the detection of early-stage cancers.
4. Biopsy and Exfoliative Cytology Examination
Biopsy is of decisive significance in determining large intestine cancer, especially early-stage cancer and polyp carcinogenesis, as well as in the differential diagnosis of lesions. It can not only clarify the nature of the tumor, histological type, and degree of malignancy but also predict prognosis and guide clinical treatment. Although exfoliative cytology has high accuracy, the sampling process is cumbersome, and obtaining satisfactory specimens is difficult. Additionally, observation requires experienced cytologists, leading to limited clinical application. Currently, it has mostly been replaced by direct endoscopic brush cytology for cytological diagnosis.
V. Others
Such as B-ultrasound, CT tomography, MRI, angiography, lymph node99mTc isotope scanning, etc., are used in the clinical diagnosis of large intestine cancer, with varying evaluation of effectiveness.
Except for early-stage large intestine cancer, which can have an insidious onset with no symptoms, advanced large intestine cancer often presents with varying degrees of clinical manifestations. At this stage, as long as vigilance is heightened, a detailed medical history is taken, a thorough physical examination is conducted, and supplementary tests such as laboratory, endoscopic, and X-ray examinations are performed, making a correct diagnosis is not difficult.
1. Medical History
A detailed inquiry into the medical history can often provide clues for the diagnosis of large intestine cancer. For individuals over middle age presenting with unexplained weight loss, anemia, changes in bowel habits, mucous stool, bloody stool, or intestinal obstruction, the possibility of large intestine cancer should be considered. To detect large intestine cancer early, regular follow-ups and re-examinations should be conducted for asymptomatic individuals with risk factors for large intestine cancer, such as a family history of large intestine cancer, a personal history of multiple colonic polyps, ulcerative colitis, Crohn's disease, chronic schistosomiasis, or those who have undergone pelvic radiotherapy or cholecystectomy.
2. Physical Examination
A comprehensive physical examination not only aids in the accurate diagnosis of large intestine cancer but also helps assess the severity of the condition, the extent of cancer invasion and metastasis, and serves as a reference for formulating an appropriate treatment plan. Particular attention should be paid to local signs such as intestinal obstruction, abdominal masses, and abdominal tenderness. Since the vast majority of large intestine cancers occur in the rectum and sigmoid colon, a digital rectal examination is indispensable. Patients presenting with hematochezia, changes in bowel habits, or stool deformation should undergo a digital rectal examination. During the examination, assess for anal or rectal stenosis, check if the examining glove is stained with blood, and if a mass is palpated, determine its location, morphology, extent, mobility at the base, and its relationship with adjacent organs.
3. Evaluation of Early Diagnosis and Population Screening for Large Intestine Cancer
As mentioned earlier, the incidence of large intestine cancer is increasing annually, with a high mortality rate, and the 5-year survival rate is closely related to the Dukes staging. Since the etiology of large intestine cancer remains unclear, improving survival rates relies on secondary prevention, namely the early diagnosis of large intestine cancer. Early diagnosis encompasses two aspects: early detection and early confirmation. Currently, with the widespread use of fiber colonoscopy, endoscopic biopsy for pathological examination has become very straightforward, making the confirmation of precancerous lesions or early-stage cancer relatively easy. However, the early detection of large intestine cancer still faces multiple challenges. Primarily, early-stage large intestine cancer often has subtle symptoms, and patients seeking medical attention usually present with advanced-stage cancer. Additionally, there is a lack of specific laboratory tests for diagnosing early-stage cancer.
Screening asymptomatic populations or monitoring individuals with a family history of large intestine cancer or confirmed precancerous lesions is an important approach to detecting early-stage cancer. Since the definitive diagnosis of cancer often relies on fiber colonoscopy and pathological biopsy, any form of screening must consider workload, economic costs, and societal feasibility. Conducting preliminary screening tests to narrow down high-risk populations can compensate for the limitations of fiber colonoscopy in application. Even from the perspective of screening efficiency alone, preliminary screening tests can enhance the detection rate of fiber colonoscopy. For example, in a screening of over ten thousand individuals, we compared the results of sigmoidoscopy alone with sequential immunochemical fecal occult blood testing followed by colonoscopy. We found that after preliminary screening, the detection rate of cancer by sigmoidoscopy increased from 0.14% to 0.43%.
As a preliminary screening test for large intestine cancer, the method must not only be sensitive and specific but also simple, easy to perform, economical, and practical. To date, various methods have been attempted for the experimental diagnosis of large intestine cancer, but the vast majority fail to meet the above requirements. This is because most diagnostic indicators only show average differences between large intestine cancer patients and control patients, yet they are not specific enough to establish a diagnostic threshold for cancer, and they are often insensitive to early-stage cancer. Based on large intestine cancer screening data worldwide, the current primary screening tests used in mass screenings are fecal occult blood tests and the recently developed rectal mucus T-antigen detection. Additionally, the use of monoclonal antibodies to detect large intestine cancer-associated antigens in blood or stool is being tested in small-scale screening populations.
There are various methods for fecal occult blood testing. Chemical occult blood tests are simple but prone to false positives (due to factors such as consuming meat, fresh fruits, vegetables, iron supplements, aspirin, etc.) and false negatives (due to prolonged stool storage, decomposition of hemoglobin in the intestinal lumen, or intake of antioxidants like vitamin C). Immunoassays represent the second-generation screening test for large intestine cancer after chemical occult blood tests, with the notable advantage of high specificity and resistance to interference from food or drugs. Early research employed agar immunodiffusion, but our application revealed that while its specificity was good, its sensitivity for cancer detection was not superior to chemical methods. Subsequently, we compared reverse indirect hemagglutination, immunolatex agglutination, and SPA co-agglutination tests. These methods all involve coating carriers with human hemoglobin antibodies. Results showed that the SPA immunoassay significantly improved the sensitivity and specificity of occult blood detection. In a screening of 8,233 cases, 934 tested positive, including 4 cases of large intestine cancer, 3 of which were early-stage. Notably, the SPA test uses Staphylococcus aureus protein A as a carrier, eliminating the need for antibody purification or complex processing. The procedure requires only mixing a drop of fecal sample with the SPA reagent, yielding stable results within 1–3 minutes, making it highly suitable for mass screening.
It is important to note that fecal occult blood tests detect large intestine cancer based on intestinal bleeding. Thus, patients with non-bleeding or intermittently bleeding tumors may be missed. Many non-neoplastic intestinal bleedings can yield false positives. In an endoscopic screening of over 3,000 individuals aged 40+, we identified 5 cases of large intestine cancer (2 early-stage), all of which tested negative for occult blood. Conversely, over 97% of occult blood-positive cases were due to non-neoplastic bleeding. Additionally, immunoassays face an optimal reaction ratio issue. Excessive blood or hemoglobin in the stool can cause false negatives, known as the "prozone" phenomenon.
To address the limitations of occult blood tests, Shamsuddin et al. in the U.S. recently proposed the rectal mucus galactose oxidase test (Shams' test) for large intestine cancer screening, based on the expression of T-antigen-like markers in cancerous and precancerous mucosal tissues. In China, we were the first to validate this method for large intestine cancer screening and adapted it for large-scale use. Results showed an 89.6% positivity rate for clinical colorectal cancer detection. In a screening of 3,820 individuals aged 40+, Shams' test was compared with the SPA immunoassay. The former yielded a 9.1% positivity rate and a 12.7% lesion detection rate, including 2 early-stage cancers and 28 adenomas, demonstrating significant complementary value to the SPA test.
Developing more sensitive and specific preliminary screening methods for large intestine cancer remains a critical challenge in its prevention. Recent reports suggest detecting ras oncogene mutations in fecal samples, but clinical application of this genetic-level finding is still premature. Current research focuses on optimizing screening protocols using existing tests. Future large intestine cancer screening may no longer rely solely on colonoscopy or occult blood-colonoscopy sequences but instead integrate and complement various assays based on their sensitivity, specificity, cost-effectiveness, patient acceptance, and societal feasibility, thereby improving screening efficacy.
bubble_chart Treatment Measures
The treatment of large intestine cancer advocates a comprehensive treatment plan centered on surgical resection. Most primary tumors can undergo radical resection, with the principle being the removal of the affected intestinal segment (including 10 cm proximal and 7 cm distal to the tumor, the corresponding mesentery, and regional lymph nodes). The specific extent of radical resection and the surgical approach depend on the location of the tumor. If radical surgery is not feasible, palliative resection can still be performed to alleviate symptoms and improve the patient's quality of life. The 5-year survival rate after surgery is closely related to the pathological stage of large intestine cancer. The 5-year survival rate for early-stage large intestine cancer can exceed 80%, while for intermediate and advanced stages, it is only around 40%.
The comprehensive treatment plan includes chemotherapy (with 5-fluorouracil as the first choice), radiotherapy, hyperthermia, etc. The efficacy rate of 5-fluorouracil for large intestine cancer is close to 20%. Combination therapies such as MF (5-fluorouracil + semustine) and MOF (mitomycin + 5-fluorouracil + vincristine) can achieve an efficacy rate of up to 30%. Recently, Southern Hospital proposed high-dose 5-fluorouracil intraperitoneal constant-temperature perfusion chemotherapy, which can improve the postoperative survival rate of large intestine cancer.
Radiotherapy is mostly suitable for fixed-position tumors in the rectum and lower sigmoid colon, with good effects on well-differentiated adenocarcinomas smaller than 5 cm in diameter. Radiotherapy is often performed preoperatively but can also be administered intraoperatively or postoperatively. Intracavitary radiotherapy has advantages such as high efficacy and fewer side effects, with a dose of approximately 10,000–15,000 cGy over 4–6 weeks. After treatment, the local symptom relief rate can reach 50–85%, generally providing 6–8 months of remission.
Hyperthermia as a conventional adjuvant treatment for large intestine cancer remains controversial, but some studies suggest that preoperative hyperthermia can increase the resection rate for intermediate and advanced-stage large intestine cancer. Additionally, electrocoagulation, cryotherapy, microwave therapy, and photodynamic therapy have shown certain effects. It is worth noting that large intestine cancer is a systemic, chronic wasting disease. To enhance tolerance to chemotherapy and radiotherapy and ensure the smooth completion of comprehensive treatment, strengthening supportive care, improving the patient's nutritional status, enhancing constitution, and thereby boosting immune function are crucial components of the comprehensive treatment plan.
Biological immunotherapy is a research direction in the treatment of large intestine cancer. For example, in addition to traditional immunotherapies using immunomodulatory agents and vaccines, recent approaches involve using membrane antigens prepared from surgically resected tumor specimens of large intestine cancer patients, encapsulated in liposomes, and injected back into the patients. This can specifically activate immune cells to kill residual cancer cells and improve the 5-year survival rate. Targeted chemotherapy and radiotherapy using monoclonal antibodies conjugated with large intestine cancer-related antigens, toxins, or radionuclides have made significant progress in animal experiments. Adoptive immunotherapy using LAK cells and tumor-infiltrating lymphocytes has been tested clinically. Furthermore, gene transfection techniques to introduce cytokines, anti-cancer genes, or heterologous antigens into tumor cells to kill or induce the killing of cancer cells provide new avenues for large intestine cancer treatment.
Large intestine cancer must be differentiated from other intestinal lesions with symptoms such as abdominal masses, abdominal colicky pain, rectal bleeding, or changes in bowel habits. These include benign tumors or polyps of the large intestine, such as adenomas, inflammatory polyps, juvenile polyps, intestinal lipomas, hemangiomas, and leiomyomas; various inflammatory diseases of the large intestine, such as ulcerative colitis, Crohn's disease, amebic colitis, schistosomiasis, intestinal tuberculosis, diverticulitis, periappendiceal inflammatory masses, radiation enteritis, and lymphogranuloma venereum; benign rectal and anal conditions such as hemorrhoids, anal fissures, and anal fistulas. Other conditions like intussusception, sigmoid fecal impaction, and rare intestinal endometriosis are also among the differential diagnoses. Since the symptoms of large intestine cancer are not specific and overlap with those of many other intestinal diseases, clinical diagnosis often adopts an active diagnostic approach, with exclusionary methods rarely used. For suspected patients, a detailed medical history followed by thorough examinations, combined with fiber colonoscopy, X-ray barium enema, and pathological biopsies, can often lead to a definitive diagnosis.
Clinically, the misdiagnosis rate of large intestine cancer is high, and most cases are confirmed at an advanced stage, which warrants vigilance and attention. The reasons for misdiagnosis and delayed diagnosis of large intestine cancer include: ① Patients' insufficient awareness of the symptoms of large intestine cancer, leading to delayed medical consultation; ② Clinicians' lack of attention to the disease, making diagnoses of hemorrhoids, enteritis, or dysentery based solely on patients' complaints of hematochezia or diarrhea without thorough history-taking and examination; ③ Relying solely on stool test results, such as the presence of phagocytes, amebic trophozoites, or dysentery bacilli in culture, without further analysis or examination; ④ Being satisfied with the discovery of benign anorectal conditions like hemorrhoids, polyps, or anal fistulas, while overlooking the possibility of coexisting rectal or intestinal cancer; ⑤ Unwillingness to perform a rectal examination or inability to correctly master the examination technique. Additionally, some large intestine cancers may present with no obvious symptoms in the early stages, and by the time symptoms appear, the disease may already be at an advanced stage.




