| disease | Myocardial Infarction |
| alias | Myocardial Infarction |
Myocardial infarction is caused by the occlusion of coronary arteries, leading to interrupted blood flow and resulting in localized necrosis of part of the myocardium due to severe, prolonged ischemia. Clinically, it presents with intense and prolonged retrosternal pain, fever, leukocytosis, accelerated erythrocyte sedimentation rate, elevated serum cardiac enzyme levels, and progressive electrocardiographic changes. It may also lead to arrhythmias, shock, or heart failure.
bubble_chart Epidemiology
This disease is common in European and American countries. According to a WHO report from 1986 to 1988, among 35 countries, Sweden, Ireland, Norway, Finland, and the United Kingdom had the highest annual mortality rates per 100,000 population due to acute myocardial infarction. The rates for males were 253.4, 236.2, 234.7, 230.0, and 229.2, respectively, and for females, 154.7, 143.6, 144.6, 148.0, and 171.3, respectively. The United States ranked in the middle, with rates of 118.3 for males and 90.7 for females. China and South Korea were at the bottom, with rates of 15.0 and 5.3 for males and 11.7 and 3.4 for females, respectively. Data from a 26-year follow-up in the United States showed that the annual incidence of this disease among people aged 35–84 was 71% for males and 22% for females. In the 55–64 age group, the rates were 91% for males and 25% for females; in the 65–74 age group, 119% for males and 51% for females; and in the 75–84 age group, 168% for males and 90% for females. Approximately 800,000 people experience myocardial infarction each year, with 450,000 cases being recurrent. In China, this disease was once extremely rare but has been gradually increasing in recent years. Data from the 1970s and 1980s in Beijing, Shanghai, Harbin, Guangzhou, Hebei, Liaoning, and Heilongjiang showed an annual incidence rate of 0.2%–0.6%, with urban areas higher than rural areas. Among these regions, North China, particularly Beijing and Tianjin, had the highest rates. In Beijing, the number of acute myocardial infarction cases admitted to 16 large and medium-sized hospitals annually has been increasing since 1972, peaking in 1979–1980, declining after 1981, and rising again from 1984. In 1991 (1,492 cases), the number was 2.47 times that of 1972 (604 cases). Similarly, in Shanghai, the number of hospitalized acute myocardial infarction patients in 10 major hospitals has been increasing since 1970, peaking in 1979–1981, declining after 1982, and rising again after 1985. In 1989 (300 cases), the number was 3.84 times that of 1970 (78 cases).
This disease is more common in males than females, with domestic data showing a ratio ranging from 1.9:1 to 5:1. Among patients, 87%–96.5% are aged 40 or older. The onset in females is about 10 years later than in males. The peak age for males is 51–60, while for females, it is 61–70. The gender disparity gradually diminishes with age. 60%–89% of patients have or had hypertension before the onset, and nearly half of the patients previously experienced colicky pain. Smokers, obese individuals, diabetics, and those with sedentary lifestyles are more susceptible. The disease occurs more frequently in spring and winter, which may be related to cold weather and significant temperature fluctuations. Most cases occur without obvious triggers, often during rest or sleep, while some are triggered by intense physical labor, emotional stress, or overeating. Additionally, shock, bleeding, tachycardia, or straining during bowel movements can also induce the condition.On the basis of coronary atherosclerosis lesions, complications such as atherosclerotic plaque rupture, hemorrhage, intravascular thrombosis, subintimal hemorrhage of the coronary artery, or persistent spasm of the coronary artery can lead to rapid and complete occlusion of the lumen. If collateral circulation between this coronary artery and other coronary arteries was previously insufficiently established, it can result in severe and prolonged ischemia of the myocardium supplied by this artery, leading to myocardial necrosis within more than one hour. On the basis of narrowed coronary artery lumens due to atherosclerosis, a sudden drop in cardiac output (due to hemorrhage, shock, or severe arrhythmia) or a sharp increase in left ventricular load (such as grade III physical activity, excessive emotional agitation, a sudden rise in blood pressure, or straining during defecation) can also cause severe and prolonged myocardial ischemia, leading to myocardial necrosis. After a heavy meal (especially one high in fat), elevated blood lipids and increased blood viscosity can slow local blood flow, making platelets more prone to aggregate and form thrombi. During sleep, increased vagal tone can cause coronary artery spasm, both of which can exacerbate myocardial ischemia and lead to necrosis. Myocardial infarction can occur in patients with frequent angina pectoris as well as in those who were previously asymptomatic.
The main hemodynamic changes involve the left ventricle, including weakened contractility, reduced compliance, and an immediate drop in stroke volume and cardiac output (often decreasing to 60–80% of the original level, or even 30–50% in cases of shock). Coronary artery blood pressure drops rapidly and begins to recover gradually after several hours. Heart rate increases, and arrhythmias may occur. The left ventricular ejection fraction decreases, end-diastolic pressure rises, and both diastolic and systolic volumes increase. Peak ejection and average ejection rates decline, and the maximum rate of pressure change (dp/dt) in the pressure curve decreases. Peripheral vascular resistance initially remains unchanged but may increase due to small artery constriction in the following hours before returning to normal or decreasing further. Venous oxygen content drops significantly, and the arteriovenous oxygen difference widens. Dyskinetic movements may appear during cardiac contraction, including akinesis (parts of the myocardium not contracting), hypokinesis (parts contracting weakly), paradoxical movement (parts bulging outward during systole), and asynchrony (disordered contraction sequence).After losing a significant portion of contractile myocardium and experiencing dyskinetic contraction, the remaining relatively normal myocardium must compensate by increasing contractile strength to maintain circulation, leading to cardiac remodeling. However, severe myocardial ischemia at this time reduces ventricular work, while hypotension further decreases coronary perfusion. Acidosis, systemic hypoxia, and arrhythmias further impair ventricular function, making compensatory mechanisms insufficient. The heart enlarges, and heart failure may even develop. Left heart failure typically occurs first, followed by right heart failure, though right ventricular myocardial infarction may present with right heart failure initially. Compensatory dilation of the left ventricle or infarction of the mitral valve papillary muscles can lead to papillary muscle dysfunction, causing mitral regurgitation, which in turn exacerbates heart failure.
Heart failure occurring during acute myocardial infarction is termed pump failure. According to the Killip classification, stage I pump failure represents compensated left heart failure, stage II is left heart failure, stage III is pulmonary edema, and stage IV is cardiogenic shock. Pulmonary edema and cardiogenic shock may occur simultaneously, representing the most severe stage of pump failure.bubble_chart Pathological Changes
During the acute phase, the myocardium exhibits extensive focal coagulative necrosis, with myocardial interstitial congestion, edema, and infiltration of numerous inflammatory cells. Subsequently, the necrotic myocardial fibers gradually dissolve and are absorbed, forming lytic foci, followed by the gradual formation of granulation tissue. If the lesion involves the pericardium, reactive pericarditis may occur; involvement of the endocardium can lead to mural thrombosis. Under the pressure within the cardiac chambers, the necrotic myocardial wall may rupture (cardiac rupture), which can occur in the free wall of the ventricle, papillary muscles, or ventricular septum.
Based on the size and distribution of the infarcted area within the myocardial wall, it is classified into three types: ① Transmural myocardial infarction: The lesion affects the entire or most of the ventricular wall, with a larger infarct size, typically over 2.5 cm in diameter. Thrombosis is commonly observed in the coronary arteries; pathological Q waves appear on the electrocardiogram; this type is the most common. ② Focal myocardial infarction: The infarcted area is smaller, distributed focally in one or multiple locations within the ventricular wall; clinically, it is often misdiagnosed and discovered during autopsy. ③ Subendocardial myocardial infarction: The infarcted foci are located within the inner half of the left ventricular wall, appearing as small foci but often widely distributed. In severe cases, subendocardial lesions may be present on all four surfaces of the left ventricular wall; pathological Q waves are generally absent on the electrocardiogram.
The necrotic tissue begins to be absorbed approximately 1–2 weeks later and gradually undergoes fibrosis, entering the chronic phase after 6–8 weeks, forming scars and healing, referred to as old or healed myocardial infarction. Larger scars may gradually protrude outward, forming ventricular aneurysms. The blood supply to the myocardium near the infarcted area gradually recovers as collateral circulation is established.
The size, extent, and severity of myocardial infarction primarily depend on the location, degree, speed of coronary artery occlusion, and the establishment of collateral circulation. Occlusion of the left anterior descending coronary artery is the most common, leading to infarction of the anterior wall of the left ventricle, apex, inferolateral wall, anterior septum, and anterior papillary muscles. Occlusion of the left circumflex coronary artery can cause infarction of the high lateral wall of the left ventricle, diaphragmatic surface, and left atrium, and may involve the atrioventricular node. Occlusion of the left coronary artery can result in infarction of the diaphragmatic surface of the left ventricle, posterior septum, and right ventricle, and may involve the sinoatrial and atrioventricular nodes. Infarction of the right ventricle and left or right atria is relatively rare. Subendocardial myocardial infarction of the left ventricle is often the result of severe lesions in all three major coronary arteries. Occlusion of the main coronary artery trunk leads to extensive infarction of the left ventricle.
During acute myocardial infarction, fresh thrombosis is often observed in the coronary arteries related to the infarcted area. Whether this is the cause or consequence of myocardial infarction remains debated. The effectiveness of early thrombolytic therapy in recent years suggests it is likely the primary cause.
bubble_chart Clinical Manifestations
Based on the clinical course and electrocardiographic manifestations, this disease can be divided into acute, subacute, and chronic late stage [third stage]. However, clinical symptoms primarily appear during the acute phase, and some patients may also exhibit certain prodromal manifestations.
(1) Prodromal Symptoms Sudden onset or more intense and frequent episodes of angina pectoris than usual, with prolonged duration, unclear triggers, and poor response to nitroglycerin. If angina is accompanied by nausea, vomiting, profuse sweating, bradycardia, acute cardiac insufficiency, severe arrhythmia, or significant blood pressure fluctuations, it may be a precursor to myocardial infarction (pre-infarction angina). If the electrocardiogram shows transient significant ST-segment elevation or depression, or inverted or elevated T-waves, there should be heightened vigilance for the possibility of imminent myocardial infarction. Timely and aggressive treatment may prevent myocardial infarction in some patients.
(2) Symptoms The severity varies depending on the size, location, progression rate of the infarction, and the patient's pre-existing cardiac function.
1. Pain This is the earliest symptom. The location and nature of the pain are similar to angina pectoris, but it often occurs at rest or during sleep. The pain is more severe, covers a wider area, and may last for hours or even days. Rest or nitroglycerin usually provides little relief. Patients often experience dysphoria, restlessness, sweating, fear, and a sense of impending doom. In China, about 1/6 to 1/3 of patients present with atypical pain characteristics or locations, such as in the upper abdomen, which may be mistaken for gastric ulcer perforation or acute pancreatitis; or in the jaw or neck, which may be misdiagnosed as bone or joint disease. Some patients, particularly those with diabetes or the elderly, may experience no pain at all and instead present with shock or acute heart failure. A few patients may remain asymptomatic throughout the entire course of the disease, with myocardial infarction only being discovered later.
2. Systemic Symptoms Primarily fever, accompanied by tachycardia, leukocytosis, and an elevated erythrocyte sedimentation rate, caused by the absorption of necrotic material. These symptoms typically appear 24–48 hours after the onset of pain, and their severity often correlates with the extent of infarction. Body temperature usually remains around 38°C, rarely exceeding 39°C, and lasts for about a week.
3. Gastrointestinal Symptoms About 1/3 of patients with pain experience nausea, vomiting, and upper abdominal distension or fullness in the early stages of the disease. These symptoms are related to vagus nerve stimulation by necrotic myocardium and reduced cardiac output leading to inadequate tissue perfusion. Intestinal distension is also common, and severe cases may present with hiccups.
4. Arrhythmias Occur in 75–95% of patients, mostly within 1–2 weeks after onset, particularly within the first 24 hours. Ventricular arrhythmias are the most common, especially ventricular premature beats. Frequent ventricular premature beats (more than 5 per minute), paired occurrences, or multiform or R-on-T phenomena on the electrocardiogram often herald the onset of ventricular tachycardia or fibrillation. Accelerated idioventricular rhythm may also occur, with ventricular rates between 50–100 beats per minute, potentially leading to isorhythmic dissociation or fusion with sinus rhythm or retrograde atrial conduction. Most episodes are transient and resolve spontaneously. Various degrees of atrioventricular block and bundle branch block are also common, with severe cases progressing to complete atrioventricular block. Supraventricular arrhythmias, such as supraventricular tachycardia, atrial flutter, or atrial fibrillation, are less frequent and mostly occur in patients with heart failure. Anterior wall myocardial infarction is more prone to ventricular arrhythmias, while inferior (diaphragmatic) wall infarction is more likely to cause atrioventricular block due to occlusion of the right coronary artery supplying the atrioventricular node. The block usually occurs above the bundle of His and has a better prognosis. In contrast, atrioventricular block in anterior wall infarction often results from simultaneous block in multiple bundle branches below the bundle of His, indicating extensive infarction and often accompanied by shock or heart failure, leading to a poorer prognosis.
5. Hypotension and Shock During the pain phase, a drop in blood pressure is common and may persist for weeks before rising again, often failing to return to previous levels. This does not necessarily indicate shock. However, if the pain is relieved but the systolic blood pressure remains below 10.7 kPa (80 mmHg), accompanied by dysphoria, pale complexion, cold and clammy skin, a thready and rapid pulse, profuse sweating, reduced urine output (<20 ml/h), mental dullness, or even syncope, these are manifestations of shock. Shock typically occurs within hours to one week after the onset of symptoms and is observed in 20% of patients. It is primarily cardiogenic, resulting from extensive myocardial necrosis (over 40%) and a sharp decline in cardiac output. Secondary factors include neurogenic peripheral vasodilation, and in some cases, hypovolemia may also contribute. Severe shock can be fatal within hours, though it generally lasts for several hours to days and may recur.
6. Heart failure Mainly acute left heart failure, which may occur within the first few days of onset or during the improvement stage of pain and shock. The incidence rate is approximately 20-48%, caused by significantly weakened cardiac contractility and reduced compliance after infarction. Patients may experience dyspnea, cough, cyanosis, dysphoria, etc. In severe cases, pulmonary edema or even right heart failure may occur, manifesting as jugular vein distension, liver swelling and pain, and edema. In cases of right ventricular myocardial infarction, manifestations of right heart failure may appear from the very beginning.
(3) Signs The cardiac dullness border may increase from grade I to grade II; the heart rate may increase or decrease, the first heart sound at the apex may weaken, and a third or fourth heart sound gallop rhythm may appear. Approximately 10-20% of patients develop pericardial friction rub 2-3 days after onset, which mostly disappears within 1-2 days, with a few cases persisting for more than a week. In cases of mitral valve papillary muscle dysfunction, a rough systolic murmur may appear at the apex. In cases of ventricular septal perforation, a loud systolic murmur may appear at the lower left sternal border. Patients with arrhythmia, shock, or heart failure will exhibit related signs and blood pressure changes.
bubble_chart Auxiliary Examination
(1) White Blood Cell Count Within the first week of onset, the white blood cell count may increase to 10,000–20,000/mm3, with neutrophils accounting for 75–90%, and eosinophils decreasing or disappearing.
(2) Erythrocyte Sedimentation Rate The erythrocyte sedimentation rate increases and may remain elevated for 1–3 weeks.
(3) Serum Enzyme Measurement Serum creatine phosphokinase (CK or CPK) appears within 6 hours of onset, peaks at 24 hours, and disappears after 48–72 hours, with a positive rate of 92.7%. Aspartate aminotransferase (AST or GOT) rises 6–12 hours after onset, peaks at 24–48 hours, and returns to normal after 3–6 days. Lactate dehydrogenase (LDH) increases 8–12 hours after onset, peaks at 2–3 days, and returns to normal after 1–2 weeks. In recent years, other enzymes such as α-hydroxybutyrate dehydrogenase (α-HBDH), γ-glutamyl transpeptidase (γ-GTP), and pyruvate kinase (PK) have also been used. Creatine phosphokinase has three isoenzymes, among which CK-MB originates from the myocardium, exhibiting extremely high diagnostic sensitivity and specificity, reaching 100% and 99%, respectively. The magnitude and duration of its elevation are often used to determine the extent and severity of infarction. Lactate dehydrogenase has five isoenzymes, among which LDH1 originates from the myocardium. It appears within hours after acute myocardial infarction, before the total lactate dehydrogenase rises, and can persist for 10 days, with a positive rate exceeding 95%.
(4) Myoglobin Measurement Measurement of urinary myoglobin excretion and serum myoglobin levels also aids in the diagnosis of acute myocardial infarction. Urinary myoglobin excretion begins 5–40 hours after infarction and persists for an average of 83 hours. The rise in serum myoglobin occurs slightly earlier than CK, around 4 hours, and its peak disappears faster than CK, with most cases returning to normal within 24 hours.
(5) Others Serum myosin light or heavy chains and serum free fatty acids both increase after acute myocardial infarction. A significant increase in serum free fatty acids is associated with a higher risk of severe ventricular arrhythmias. Additionally, due to the stress response during acute myocardial infarction, blood glucose may rise, and glucose tolerance may temporarily decrease, returning to normal after approximately 2–3 weeks.
[Electrocardiogram and Vectorcardiogram Examination]
The electrocardiogram shows progressive and characteristic changes, which are highly valuable for diagnosis, localization, assessment of the extent, and monitoring the progression of the condition. The electrocardiographic waveform changes include three types:
1. Waveform of the Necrotic Zone Leads facing the necrotic myocardium show deep and wide Q waves.
2. Waveform of the Injury Zone Leads facing the area surrounding the necrotic zone display elevated ST segments.
3. Waveform of the Ischemic Zone Leads facing the periphery of the injury zone show inverted T waves.
The typical electrocardiographic evolution is as follows: - **Acute Phase (Onset):** Leads facing the infarcted area show abnormal Q waves and markedly elevated ST segments, with the latter arched upward and merging with the T wave to form a monophasic curve, while the R wave decreases or disappears. Leads opposite the infarcted area show increased R waves and depressed ST segments. - **Subacute Phase (Days to 2 Weeks After Onset):** In leads facing the infarcted area, the ST segment gradually returns to baseline, and the T wave becomes flat or markedly inverted. In leads opposite the infarcted area, the T wave increases. - **Chronic Phase (Weeks to Months After Onset):** The T wave may become V-shaped and inverted, with symmetrical limbs and a sharp trough. The abnormal Q wave often persists permanently, while the T wave may recover over months to years (Figure 6).
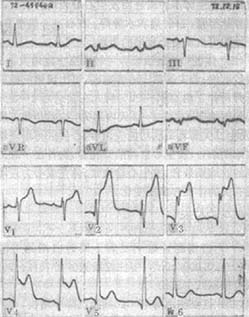
Figure 6 Electrocardiogram of Acute Anterior Wall Myocardial Infarction
The QRS complex in leads V3 and V4 shows a qR pattern with significant ST-segment elevation, while lead V2 displays a qRS pattern with marked ST-segment elevation. Additionally, the ST segment in lead V1 is also elevated.
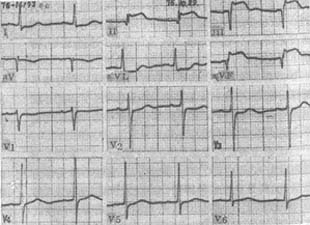
Figure 7 Electrocardiogram of Acute Inferior Myocardial Infarction
The illustration shows Qr pattern with ST-segment elevation in leads III and aVF, and qRsr′ pattern with ST-segment elevation in lead II. ST-segment depression is observed in leads I and aVL.
In the earliest stage before the appearance of abnormal Q waves and ST-segment elevation, the ECG may show no abnormalities or exhibit unusually tall T waves with asymmetrical limbs. Multifocal myocardial infarction may lack typical ECG manifestations. When combined with bundle branch block, especially left bundle branch block, the ECG may not necessarily reflect acute myocardial infarction. Recurrent acute myocardial infarction in the same location often presents with atypical ECG findings.
The basis for ECG localization of infarction sites is listed in Table 1.
Table 1 ECG Localization Diagnosis of Myocardial Infarction
| Leads | Anteroseptal | Localized Anterior Wall | Anterolateral Wall | Extensive Anterior Wall | Inferior Wall* | Inferoseptal | Inferolateral Wall | High Lateral Wall** | True Posterior Wall*** |
| V1 | + | + | + | ||||||
| V2 | + | + | + | ||||||
| V3 | + | + | + | + | |||||
| V4 | + | + | |||||||
| V5 | + | + | + | + | |||||
| V6 | + | + | |||||||
| V7 | + | + | + | ||||||
| V8 | + | ||||||||
| aVR | |||||||||
| aVL | ± | + | ± | - | - | - | + | ||
| aVF | … | … | … | + | + | + | - | ||
| Ⅰ | ± | + | ± | - | - | - | + | ||
| II | … | … | … | + | + | + | - | ||
| III | … | … | … | + | + | + | - |
[Note] "+" indicates positive changes, representing typical Q waves, ST-segment elevation, and T-wave inversion.
"-" indicates negative changes, representing changes antagonistic to the above.
"±" indicates possible positive changes.
"…" indicates possible negative changes.
*Refers to the diaphragmatic surface. Right ventricular myocardial infarction is difficult to diagnose via ECG, but ST-segment elevation in lead CR4 (or V4R) can serve as a reference indicator for inferior wall myocardial infarction extending to the right ventricle.
**Positive changes are observed in leads V5, V6, and V7 at 1–2 intercostal spaces higher.
***Increased R waves in leads V1 and V2.
Clinical data in China show that myocardial infarctions in the inferior wall, anterior septum, and localized anterior wall are the most common.
Atypical ECG changes in myocardial infarction include:
(1) Subendocardial myocardial infarction: ECG changes resemble those of grade III myocardial ischemia. Except for lead aVR, which shows ST-segment elevation, all other leads generally exhibit ST-segment depression, biphasic or inverted T waves (initially negative then positive), and decreased R waves. These changes may persist for weeks or months, or even long-term (Figure 8). This is also referred to as non-Q wave myocardial infarction.
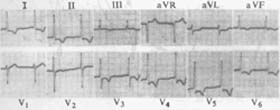
Figure 8 ECG of subendocardial myocardial infarction
Shows ST-segment depression in all leads except V1 and III, with ST-segment elevation in lead aVR.
(2) Myocardial Infarction with Only T Wave Changes When the electrocardiogram shows progressive T wave inversion from shallow to deep over several weeks, accompanied by clinical manifestations of myocardial infarction, followed by gradual recovery of the T waves, some believe it can be concluded that the patient has experienced a myocardial infarction. However, since many factors can influence T wave changes, the diagnosis should also consider clinical manifestations and changes in blood cardiac enzyme levels.
(3) Papillary muscle infarction When the anterolateral papillary muscle of the mitral valve is infarcted, leads I, aVL, and V4 to V6 show abnormal changes; when the posteromedial papillary muscle is infarcted, leads II, III, aVF, and V1 to V4 show abnormal changes. In acute infarction or rupture, the manifestations are mostly J-point depression, upward convex ST segment, inverted T wave, or J-point depression with downward concave and depressed ST segment; in old infarction, the manifestations are mostly J-point depression, downward concave ST segment, and upright T wave. However, the diagnostic specificity of these patterns is not high.
There have been attempts to place dozens of electrodes on the precordial area to record multiple precordial ECG leads for isopotential precordial mapping, measuring the degree of ST-segment elevation and the presence of Q waves to create a map. The sum of ST-segment elevation or depression and Q-wave presence reflects the time-potential changes of the cardiac cycle on the chest wall, which is considered more accurate than conventional ECG in assessing the extent and progression of acute anterior wall myocardial infarction. However, the procedure is cumbersome and not sensitive for localizing inferior wall myocardial infarction, so it is not commonly used.
Atrial infarction is manifested on ECG by changes such as increased and widened P waves, notching, and Ta wave depression, but it is extremely rare. Vectorcardiogram shows changes in the QRS loop, the appearance of ST vectors, and changes in the T loop. The changes in the QRS loop are the most diagnostically valuable. Because necrotic myocardial cells cannot depolarize and generate the expected electrical forces, the resultant vector during ventricular depolarization deviates away from the infarcted area. The resulting QRS loop, especially the initial vector, points in the direction antagonistic to the infarcted area, and this change in initial vector orientation is significant for localization. The appearance of ST vectors is manifested as an open QRS loop, where the endpoint does not return to the starting point. The line from the starting point to the endpoint of the QRS loop represents the direction of the ST vector, pointing toward the infarcted area. ST vectors usually disappear within 1–2 weeks. Changes in the T loop mainly manifest as the maximum vector being antagonistic to the maximum mean QRS vector or an increased QRS-T angle, with a T-loop length/width ratio < 2.6:1, and equal speeds of the centrifugal and centripetal limbs of the T loop. These changes may last for months to years before disappearing (Figure 9-10).

Figure 9 Vectorcardiogram of anteroseptal myocardial infarction
Horizontal plane QRS loop runs counterclockwise, mainly in the left posterior quadrant, with no anterior vector in the initial portion; the 0.01-second vector points left posteriorly, and the 0.02-second vector also points left posteriorly. Right sagittal plane QRS loop forms a figure-8, first running counterclockwise then clockwise, mainly located in the to be decocted later quadrant, with no anterior vector in the initial portion. Frontal plane QRS loop runs clockwise, with the initial portion pointing left and downward.

Figure 10 Vectorcardiogram of inferior wall myocardial infarction
Right sagittal plane QRS loop forms a figure-8, first running clockwise then counterclockwise, mainly located in the superior anterior quadrant, with an initial upward vector lasting >0.025 seconds. Frontal plane QRS loop runs clockwise, with the entire loop mainly in the left superior quadrant, initial upward vector lasting >0.025 seconds, maximum vector located at -18°, and a "notch" on the centripetal limb. Horizontal plane QRS loop runs counterclockwise, located in the left anterior and left posterior quadrants. Right sagittal and frontal plane T loops both point upward.
Vectorcardiogram may be more sensitive than ECG in diagnosing myocardial infarction but is not more specific, requiring comprehensive consideration with clinical data.
[Radionuclide myocardial imaging]Myocardial hotspot imaging with 99mTc-pyrophosphate shows that the uptake rate in necrotic myocardium peaks in most patients within 48–72 hours and decreases after 6–7 days. The positive rate ranges from 66% (subendocardial myocardial infarction) to 89% (transmural myocardial infarction). For myocardial cold spot imaging using 99mTc-MIBI or 201Tl, the former achieves a positive rate of 100% when performed 30–80 minutes after onset, while the latter achieves nearly 100% positivity within 6 hours of onset, declining after 24 hours. Gated blood pool imaging with 99mTc-labeled red blood cells or albumin allows observation of ventricular wall motion and left ventricular ejection fraction, aiding in assessing ventricular function, diagnosing post-infarction wall motion abnormalities, and identifying ventricular aneurysms (Figure 11).

Figure 11 99mTechnetium-labeled red blood cell cervical orifice circuit cardiac blood pool imaging
The left column shows end-systole, and the right column shows end-diastole. The first row is the right anterior oblique 20° view, the second row is the anterior view, the third row is the left anterior oblique 45° view, the fourth row is the left anterior oblique 70° view, and the fifth row is the left lateral view. The left ventricle is enlarged with irregular morphology, showing no movement or slight bulging in the anterolateral wall and apex during systole, suggesting possible ventricular aneurysm formation.
[Echocardiography]
Two-dimensional, Doppler, and M-mode echocardiography can measure the left ventricular ejection fraction, ventricular volume, and wall motion, or additional dobutamine stress tests may assist in diagnosis.
Other examinations such as magnetic resonance imaging are also helpful for diagnosis.
Based on typical clinical manifestations, characteristic ECG changes, and laboratory findings, diagnosing this disease is not difficult. For painless patients, diagnosis is more challenging. Any elderly patient who suddenly experiences shock, severe arrhythmia, heart failure, upper abdominal distension and fullness, pain, or vomiting without a clear cause, or a hypertensive patient whose blood pressure suddenly drops without explanation, or postoperative shock without bleeding or other identifiable reasons, should consider the possibility of myocardial infarction. Additionally, elderly patients with severe and prolonged chest tightness or chest pain, even without characteristic ECG changes, should also be suspected of this condition. In such cases, it is advisable to manage them as acute myocardial infarction initially and conduct repeated ECG monitoring and cardiac enzyme tests within a short period to confirm the diagnosis.
bubble_chart Treatment Measures
(1) Prevention of coronary heart disease: Refer to the section on "Occult Coronary Atherosclerotic Heart Disease" in this chapter. Long-term oral administration of low-dose aspirin (0.05–0.3g/day) or dipyridamole (50mg three times daily) to inhibit platelet aggregation and adhesion is considered effective in preventing the recurrence of myocardial infarction.
(2) Timely and active treatment of prodromal symptoms: The appearance of prodromal symptoms may indicate an impending myocardial infarction. It is advisable to recommend hospitalization for the patient and to promptly and aggressively manage the condition using measures for treating myocardial infarction, which may reduce the likelihood of infarction in these patients.
(3) Treatment of acute myocardial infarction: During this period, the treatment principles are to protect and maintain cardiac function, salvage濒死的心肌, prevent the expansion of the infarction, reduce the area of myocardial ischemia, and promptly manage any complications. The goal is to not only help the patient survive the critical acute phase but also preserve as much functional myocardium as possible for a more effective quality of life post-recovery.
1. Pre-hospital management: Approximately two-thirds of patients with acute myocardial infarction die before reaching the hospital. Therefore, shortening the time from symptom onset to hospitalization and providing active treatment during this period are crucial for saving lives. For severely ill patients, emergency care should be administered on-site until the patient's condition stabilizes enough for transfer to a hospital. Ambulances transporting patients should be equipped with monitoring devices to allow continuous observation of the patient's condition during transit and prompt intervention if needed.
2. Monitoring and general treatment:
(1) Rest: Patients should be confined to bed rest in a "coronary care unit," with a quiet environment, limited visitation, and avoidance of adverse stimuli.
(2) Oxygen therapy: Intermittent or continuous oxygen administration via nasal cannula or face mask for the first 2–3 days.
(3) Monitoring: Continuous ECG, blood pressure, and respiratory monitoring for 5–7 days, with hemodynamic monitoring if necessary. Close observation of the patient's condition provides an objective basis for timely treatment. Monitoring personnel must work with utmost responsibility, ensuring no significant changes are overlooked while maintaining the patient's rest and comfort.
(4) Nursing care: Complete bed rest is required during the first week, with intensive nursing care. Nursing staff must wholeheartedly assist patients with eating, washing, turning, and using the bedpan. Patients should avoid overeating; food should be easily digestible, low in fat, and non-gas-producing, with limited sodium intake while ensuring adequate calories and nutrition. Bowel movements should be kept regular, but straining should be avoided. Laxatives may be given if constipation occurs. In the second week, patients may sit up in bed, gradually move to standing by the bed, and take slow walks indoors. Some advocate for early ambulation (within the first week), but patients with severe conditions or complications should not have their bed rest period shortened excessively.
3. Pain relief: Administer meperidine (pethidine) 50–100mg intramuscularly or morphine 5–10mg subcutaneously, repeatable every 4–6 hours. Alternatively, nitroglycerin 0.3mg or isosorbide dinitrate 5–10mg may be given sublingually. Nitroglycerin 1mg dissolved in 100ml of 5% glucose solution can be infused intravenously at 10–50µg/min, or isosorbide dinitrate 10mg in 100ml of 5% glucose solution at 30–100µg/min, with careful blood pressure monitoring. Chinese herbal medicines such as Su Bing Drop Pills, Styrax Pills, Coronary Heart Styrax Pills, or Kuanxiong Pills may be taken sublingually or orally. Alternatively, compound Salvia injection (2–4ml) may be administered intravenously with 40ml of 50% glucose solution, or 8–16ml may be infused with 500ml of 50% glucose solution or low-molecular-weight dextran.
In recent years, some have proposed the use of beta-blockers such as metoprolol (15mg intravenous injection followed by oral administration of 50mg four times a day for 2 days, then adjusted to 100mg twice a day for 3 months), propranolol, atenolol, and timolol. It is believed that these medications can provide pain relief and improve prognosis for patients with anterior wall infarction who have higher blood pressure and faster heart rates. However, close monitoring of blood pressure, heart rate, and cardiac function is essential during the treatment process.
4. Reperfusion of Myocardium: Drugs that dissolve coronary artery thrombi should be used as early as possible to restore myocardial perfusion, salvage濒死 myocardium, reduce the size of myocardial infarction, protect ventricular function, and alleviate pain. Suitable for: ① onset ≤6 hours, ② ST-segment elevation ≥0.2mV in two or more adjacent leads, ③ age ≤70 years, and no contraindications such as recent active bleeding, apoplexy, bleeding tendency, diabetic retinopathy, severe hypertension, or severe liver and kidney dysfunction.
⑴ Intravenous thrombolytic therapy: Options include: ① Urokinase (most commonly used in China), 100–150U infused over 1/2–1 hour; ② Streptokinase, 1–1.5 million U infused over 1 hour (with 2.5–5mg dexamethasone to prevent shiver fever reaction); ③ Recombinant tissue-type plasminogen activator (rtPA), 10mg bolus followed by 50mg over 1 hour, then 40mg over 2 hours; ④ Single-chain urokinase-type plasminogen activator (SCUPA), 20mg bolus followed by 60mg over 1 hour; ⑤ Anisoylated plasminogen streptokinase activator complex (APSAC), 30mg single bolus. Before administration, take aspirin 300mg/day, then reduce to 50mg/day after 3 days for long-term use. After thrombolysis, measure clotting time and fibrinogen every 4–6 hours. When clotting time returns to 1.5–2.0 times the normal control and fibrinogen >1000mg/L, administer heparin 5000U IV, followed by 500–1000U/h IV infusion, adjusting the dose to maintain clotting time at twice the normal value, and discontinue after 5–7 days. Monitor closely for bleeding tendencies during treatment. Indicators of successful reperfusion include: ① relief of chest pain within 2 hours, ② resolution of ST-segment elevation or >50% ST-segment reduction every 30 minutes, ③ peak CPK-MB levels occurring within 14 hours of onset, and ④ occurrence of ventricular arrhythmias or conduction block within 2 hours.
⑵ Intracoronary thrombolytic therapy: First perform selective coronary angiography, then inject 2000µg nitroglycerin. For urokinase, inject 30,000U initially, followed by 4000–8000U/min, with angiography every 10–15 minutes. If the vessel recanalizes, halve the dose and maintain for 1/2–1 hour. For streptokinase, inject 30,000U initially, followed by 2000–4000U/min, and maintain for 1/2–1 hour after recanalization. For rtPA, inject 10mg initially, followed by 40mg over 30 minutes, then 50mg over 1 hour. This method is more effective with smaller doses but requires angiography equipment and expertise. The preparation and procedure may delay treatment, making it less commonly used than intravenous administration currently.
Thrombolytic therapy achieves an average coronary artery recanalization rate of about 75%. For non-recanalized vessels, PTCA can be used to dilate and reopen them. In recent years, some advocate direct PTCA for coronary recanalization without prior thrombolytic drugs, claiming a recanalization rate of up to 90%.
5. Elimination of Arrhythmias (see "Arrhythmias").
⑴ Ventricular arrhythmias: Some advocate the immediate intramuscular injection of 200–250 mg of lidocaine after the onset of myocardial infarction to prevent ventricular arrhythmias. For frequent ventricular premature beats or ventricular tachycardia, 50–100 mg of lidocaine should be administered intravenously (if ineffective, it can be repeated after 5–10 minutes). After control is achieved, lidocaine should be administered via intravenous drip at a maintenance rate of 1–3 mg per minute (100 mg of lidocaine in 100 ml of 5% glucose solution, infused at 1–3 ml/min). Once the condition stabilizes, consideration may be given to switching to oral medications such as mexiletine 150–200 mg, procainamide 250–500 mg, bretylium 100–200 mg, disopyramide 100–200 mg, tocainide 400–600 mg, or quinidine 0.2 g, administered every 6 hours for maintenance. In the event of ventricular fibrillation, immediate DC cardioversion should be performed using the most appropriate energy level (generally 300 J), aiming for successful defibrillation in a single attempt. If electrical defibrillation is unavailable, immediate external cardiac massage and mouth-to-mouth artificial respiration should be initiated, along with intracardiac injection of 100–200 mg of lidocaine, 200–300 mg of procaine, or 250 mg of bretylium, followed by other cardiac resuscitation measures (refer to Chapter 6, "Cardiac Arrest and Sudden Cardiac Death"). Accelerated idioventricular rhythm generally requires no intervention, but if hemodynamic instability arises due to the loss of atrial contribution to ventricular filling, atropine may be used to accelerate the sinus rhythm and control heart rate. Only in rare cases is artificial cardiac pacing or drugs to suppress ectopic rhythms necessary.
⑵ Atrioventricular block: For grade III (including those estimated to potentially progress to grade III) and grade II type II (Mobitz type II) atrioventricular block, temporary artificial cardiac pacing should be used and removed once the condition improves. If the block becomes persistent, a permanent implantable pacemaker should be installed later. For grade I and grade II type I (Wenckebach phenomenon) atrioventricular block, treatment with corticosteroids, atropine, isoproterenol, or ephedrine may be initiated based on the patient's condition, with close monitoring of progression.
⑶ Bradyarrhythmias: For various bradyarrhythmias, including sinus, atrioventricular junctional, and ventricular types, treatment options include atropine, isoproterenol, ephedrine, or sodium lactate (intravenous injection or infusion). Previously, atropine was considered more appropriate, while isoproterenol could be used if hypotension coexisted. However, the latter also enhances myocardial contractility, increasing oxygen consumption and potentially inducing arrhythmias. Recent studies suggest that atropine, while increasing heart rate, also raises myocardial oxygen demand and may cause severe arrhythmias, thus requiring cautious use. If these drugs are ineffective or cause significant side effects, artificial cardiac pacing may be considered.
⑷ Supraventricular tachyarrhythmias: Such as sinus tachycardia, frequent atrial premature beats, paroxysmal supraventricular tachycardia, atrial flutter, and atrial fibrillation, may be treated with β-blockers, digitalis, verapamil, amiodarone, quinidine, procainamide, or antazoline. If the latter three treatments fail, synchronized direct current cardioversion or artificial cardiac pacing may be considered (see Chapter 7, "Artificial Cardiac Pacing and Cardioversion") to minimize the duration of the tachyarrhythmia.
⑸ Cardiac arrest: Immediate external cardiac massage and artificial respiration should be performed, along with intracardiac injection of adrenaline, isoproterenol, sodium lactate, and atropine, followed by other cardiac resuscitation measures (see Chapter 6, "Cardiac Arrest and Sudden Cardiac Death").
6. Treatment of shock (also see Chapter 4, "Shock" and Chapter 2 of Part 10, "Cardiovascular System Function Monitoring").
⑴ General management and monitoring: Administer oxygen, keep warm, and closely monitor blood pressure, urine output, central venous pressure, pulmonary "capillary" pressure (pulmonary wedge pressure), and cardiac output, adjusting treatment measures as needed.
⑵ Blood volume replenishment: Approximately 20% of patients experience hypovolemia due to vomiting, sweating, fever, diuretic use, or lack of oral intake, requiring volume replacement while avoiding excessive infusion that may lead to heart failure. Fluid administration should be guided by hemodynamic monitoring. If central venous pressure is low (49–98 Pa or 5–10 cmH2O) and pulmonary wedge pressure is below 0.8–1.6 kPa (6–12 mmHg) with low cardiac output, indicating hypovolemia, low-molecular-weight dextran or 10% glucose solution may be infused intravenously. If central venous pressure rises above 196 Pa (20 cmH2O) or pulmonary wedge pressure exceeds 2.0–2.7 kPa (15–20 mmHg), infusion should be stopped.
(3) Application of vasoconstrictors: When systolic blood pressure is below 10.7 kPa (80 mmHg), blood pressure does not rise after intravenous infusion, and pulmonary wedge pressure and cardiac output are normal, vasoconstrictors can be selected: ① Dopamine: 10–30 mg added to 100 ml of 5% glucose solution for intravenous drip, which can also be administered simultaneously with metaraminol. ② Dobutamine: 20–25 mg dissolved in 100 ml of 5% glucose solution, administered intravenously at a dose of 2.5–10 µg/(kg·min). Its effects are similar to dopamine, but it has a stronger effect on increasing cardiac output, a milder effect on accelerating heart rate, and no significant vasodilatory effect on renal blood vessels. ③ Metaraminol (Aramine): 10–30 mg added to 100 ml of 5% glucose solution for intravenous drip, or 5–10 mg administered intramuscularly. However, it is less effective for patients who have been taking guanethidine or reserpine long-term. ④ Norepinephrine: Its effects are similar to metaraminol but faster, stronger, and shorter-lasting. It remains effective for patients who have been taking guanethidine or reserpine long-term. 0.5–1 mg (approximately equivalent to 1–2 mg of the bitartrate salt) is added to 100 ml of 5% glucose solution for intravenous drip. Extravasation can easily cause local injury and necrosis. If 2.5–5 mg of phentolamine is added simultaneously, the local vasoconstrictive effect can be mitigated.
(4) Application of vasodilators: If blood pressure still does not rise after the above treatments, and pulmonary wedge pressure increases, cardiac output decreases, or peripheral vasoconstriction leads to increased total resistance, the diseased left ventricle faces high impedance, increased tension, and elevated oxygen consumption, which will worsen the degree of shock. The patient may exhibit cold extremities and cyanosis. At this point, vasodilators can be used to reduce peripheral resistance and cardiac afterload, lower left ventricular ejection resistance, enhance contractile function, thereby increasing cardiac output and improving shock conditions.
Vasodilators should be used cautiously under strict hemodynamic monitoring. Options include sodium nitroprusside (15–400 µg/min IV drip), phentolamine (0.25–1 mg/min IV drip), isosorbide dinitrate (2.5–10 mg sublingually multiple times), or nifedipine (10–20 mg orally multiple times).
(5) Use of cardiac glycosides and adrenocortical hormones: There are differing opinions on whether these two types of drugs should be used in acute myocardial infarction complicated by shock. Some believe cardiac glycosides can still be used when the heart is enlarged, while adrenocortical hormones only show effects at extremely high doses.
(6) Correction of acidosis and electrolyte imbalances, prevention of cerebral ischemia, and protection of renal function: Patients with severe or prolonged shock often have acidosis, which affects the efficacy of vasoactive drugs. Intravenous drips of 5% sodium bicarbonate, 11.2% sodium lactate solution, or 3.63% tromethamine (THAM) can be administered, with dosage adjustments based on blood pH or CO₂ combining power measurements. Special attention should be paid to correcting hypokalemia and hypochloremia. Cerebral ischemia should be avoided, and renal function should be protected.
(7) Assisted circulation and surgical intervention: If the above treatments are ineffective, some advocate the use of intra-aortic balloon counterpulsation therapy or, under counterpulsation support, selective coronary angiography followed by necrotic myocardium resection and coronary artery bypass surgery, which may save the patient's life.
(8) Right ventricular myocardial infarction complicated by shock: Hemodynamic tests often show increased central venous pressure, right atrial and right ventricular filling pressures, while pulmonary wedge pressure and left ventricular filling pressure remain normal. Treatment involves volume expansion, up to 4000–6000 mL over 24 hours, to increase right ventricular end-diastolic volume and the right atrial-left atrial pressure gradient, allowing blood to pass through the low-resistance pulmonary vascular bed, thereby increasing left ventricular filling pressure, cardiac output, and arterial pressure. However, pulmonary wedge pressure should be maintained below 2.0–2.7 kPa (15–20 mmHg) during fluid administration.
7. Treatment of heart failure: The main focus is on treating acute left heart failure (see Chapter 2, "Cardiac Insufficiency"). The primary treatments include morphine or pethidine and diuretics. Vasodilators to reduce left ventricular afterload or dobutamine therapy may also be considered. Digitalis drugs may cause ventricular arrhythmias, and early-stage heart failure is mainly due to myocardial congestion and edema leading to decreased compliance, with no increase in left ventricular end-diastolic volume. Therefore, they should only be used in mild heart failure cases and avoided within 24 hours of infarction onset. Diuretics should be used cautiously in patients with right ventricular infarction.
8. Other treatments: The following therapies may help prevent infarct expansion, reduce ischemic areas, and accelerate healing, but their efficacy is not yet fully established or remains controversial. They may be considered based on the patient's specific condition.
⑴ Drugs to promote myocardial metabolism: Vitamin C (3-4g), coenzyme A (50-100U), sodium inosinate (200-600mg), cytochrome C (30mg), vitamin B6 (50-100mg), etc., are added to 500ml of 5-10% glucose solution for slow intravenous drip, once/day, with two weeks as one course of treatment.
⑵ Polarized solution therapy: 1.5g of potassium chloride and 8U of regular insulin are added to 500ml of 10% glucose solution for intravenous drip, 1-2 times/day, with a course of 7-12 days. This promotes myocardial uptake and metabolism of glucose, facilitates potassium ions entering cells, restores the polarization state of the cell membrane, thereby aiding normal cardiac contraction, reducing arrhythmias, and helping the elevated ST segment on the ECG return to baseline.
⑶ Low-molecular-weight dextran or hydroxyethyl starch plasma substitute, 250-500ml intravenous drip, once/day, with a course of two weeks. This reduces red blood cell aggregation, lowers blood viscosity, and helps improve microcirculatory perfusion.
⑷ Hyaluronidase: First, perform an intradermal test with 150U. If negative, administer an intravenous bolus of 500U/kg. After the first dose, give the same dose again at 2-6 hours, then every 6 hours for a total of 42 hours. Early application after onset may accelerate inflammation absorption and reduce infarct size.
⑸ Glucocorticoids: A single intravenous infusion of methylprednisolone (25mg/kg) within 4 hours of onset to stabilize lysosomal membranes and reduce lysosomal enzyme release, potentially preventing infarct expansion.
⑹ Counterpulsation: Counterpulsation increases diastolic pulse pressure without increasing left ventricular systolic load, helping to enhance coronary pulse perfusion. Intra-aortic balloon counterpulsation is invasive, while external counterpulsation is non-invasive. The latter is administered 1-2 times/day, 1-2 hours each time, for about 7 days.
⑺ Anticoagulation therapy: Consider for extensive or recurrent infarction without thrombolytic therapy, or for those with infarction precursors and hypercoagulability. Contraindicated in cases of bleeding, bleeding tendency, history of bleeding, severe liver or kidney dysfunction, active peptic ulcer, severe hypertension, or recent surgery with unhealed wounds. Start with heparin 5000-7500U intravenous drip every 6 hours or 10,000U deep intramuscular injection every 8 hours for 2 days, maintaining clotting time at 2-2.5 times the normal control. Simultaneously, administer oral warfarin with an initial dose of 15-20mg, 5-10mg on the second day, then 2.5-5mg/day for maintenance; or dicoumarol with an initial dose of 200mg, 100mg on the second day, then 25-75mg/day for maintenance; or phenindione with an initial dose of 200-300mg, then 50-100mg/day for maintenance. Maintain prothrombin time at about twice the normal control, with a course of at least 4 weeks. If bleeding occurs, discontinue treatment immediately. For heparin-induced bleeding, administer an equal dose of protamine intravenously; for oral anticoagulant-induced bleeding, give vitamin K1 intravenously, 20mg/dose; blood transfusion if necessary.
⑻ Others: Beta-blockers are used for anterior wall infarction with tachycardia and hypertension, reducing mortality. Preferentially use cardioselective agents like metoprolol or atenolol. Calcium antagonists (e.g., diltiazem) and angiotensin-converting enzyme inhibitors (e.g., captopril) have also been used.
9. Traditional Chinese Medicine (TCM) Treatment: TCM formulas such as Cold-Extremities Decoction (prepared aconite root, dried ginger, prepared licorice root), Ginseng Decoction alone, or Ginseng and Aconite Decoction, used for "restoring yang to save from collapse," have shown efficacy in treating this condition with hypotension or shock. For patients with yin deficiency manifestations, Pulse-Reinforcing Powder (ginseng, schisandra fruit, ophiopogon tuber) may be used. These formulas are also available as injections for emergency use.
10. Treatment of Complications: For embolism, use thrombolytic or anticoagulant therapy. Post-myocardial infarction syndrome can be treated with glucocorticoids, aspirin, or indomethacin. Shoulder-hand syndrome may be managed with physiotherapy or physical rehabilitation.
Concurrent ventricular septal perforation, acute mitral valve insufficiency, or ventricular wall aneurysm can lead to severe hemodynamic changes or arrhythmias, and active surgical treatment should be adopted. Most of these patients are in a state of circulatory insufficiency. First, measures of assisted circulation should be used to improve the circulatory condition, while necessary preoperative examinations are conducted to understand the coronary artery lesions and myocardial lesions. Then, surgical repair of the ventricular septal perforation, replacement of the artificial mitral valve, resection of the infarcted myocardium or ventricular wall aneurysm should be performed, along with coronary artery bypass grafting to improve myocardial blood supply. However, acute free ventricular wall rupture often lacks the opportunity for surgical rescue.
11. Rehabilitation therapy Before discharge, carefully conducting exercise electrocardiogram stress tests, radionuclide or ultrasound left ventricular ejection fraction measurements, and selective coronary angiography can help in selecting further treatment measures (medication choice, PTCA or CABG) and planning rehabilitation therapy. The latter involves a specialized physician arranging appropriate exercises (walking, gymnastics, Tai Chi, etc.) based on the patient's cardiac function and physical condition to promote physical recovery.
The prognosis is related to the size of the infarction, the development of collateral circulation, and the timeliness of treatment. In the past, the mortality rate for hospitalized patients during the acute phase was generally around 30%. With intensive care treatment, this rate has decreased to about 15%, and with the development of thrombolytic therapy, it has further dropped to below 10%. During the acute phase, the mortality rate is highest in the first week of onset. Patients who develop heart failure, severe arrhythmias, or shock have an especially high mortality rate, with shock patients facing a mortality rate as high as 80%. Long-term follow-up data from Beijing, China, on myocardial infarction patients show that 53.4% of patients were able to return to some level of work, with 45.6% resuming work within six months after the illness. Among discharged patients, deaths due to cardiac causes were 7.7% in the first year, 3.7% in the second year, 3.0% in the third year, 2.7% in the fourth year, 1.4% in the fifth year, 3.4% in the sixth year, and 1.1% in the seventh year.
(1) Papillary muscle dysfunction or rupture The papillary muscles (mainly the mitral valve papillary muscles) lose their contractility or rupture due to ischemia, necrosis, etc., resulting in mitral valve insufficiency. A loud blowing systolic murmur is heard at the apex and is prone to cause heart failure.
(2) Cardiac rupture A rare but serious complication in the early stage, often occurring within one week of onset. Most cases involve rupture of the free wall of the ventricle, leading to sudden death due to pericardial effusion and acute cardiac tamponade. Occasionally, rupture and perforation of the ventricular septum occur, accompanied by a loud systolic murmur at the fourth intercostal space along the left sternal border, often with tremor, which can lead to heart failure and rapid death.
(3) Ventricular aneurysm The incidence reported in domestic data is 20%, while clinical data show 28%. It forms when the infarcted ventricular wall bulges outward under the influence of intraventricular pressure. It is seen in patients with larger myocardial infarctions and is often detected several weeks after onset. Physical examination may reveal an enlarged right cardiac border, widespread cardiac pulsation, and possibly a systolic murmur. When mural thrombosis occurs, heart sounds are weakened. The ECG shows persistent ST-segment elevation. X-ray examination may reveal localized bulging of the cardiac border, and fluoroscopy or kymography may show weakened or paradoxical pulsation in that area. Selective left ventriculography and gated radionuclide cardiac blood pool imaging can demonstrate the aneurysm (Figures 1, 2, 3). Echocardiography can reveal abnormal pulsations of the ventricular aneurysm (Figure 1). Complications of ventricular aneurysm include heart failure, arrhythmias, or embolism, but the risk of rupture is low after myocardial infarction healing.
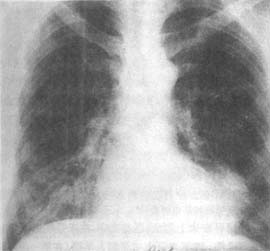
Figure 1 Frontal plain film of left ventricular aneurysm
The image shows a spherical protrusion on the left cardiac border
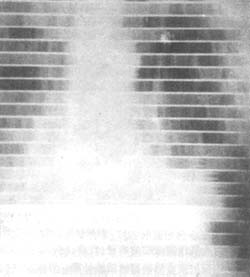
Figure 2 Frontal kymogram of left ventricular aneurysm (same patient as in Figure 1)
The image shows a large pulsation amplitude at the spherical protrusion on the left cardiac border
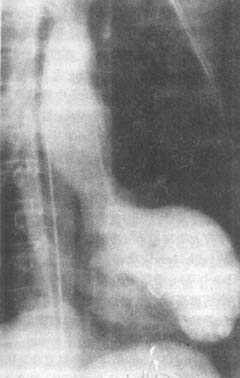
Figure 3 Right anterior oblique view of left ventriculography in left ventricular aneurysm
The image shows outward bulging of the left cardiac border during systole, with the cavity filled with contrast agent
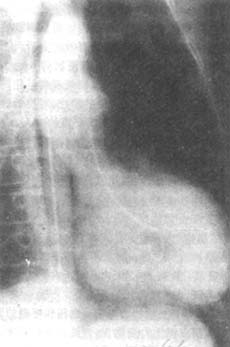
Figure 4 Right anterior oblique view of left ventriculography in left ventricular aneurysm
The image shows the left ventricular cavity filled with contrast agent during diastole, with little change in the left cardiac border compared to systole
|
|
|
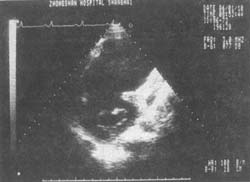
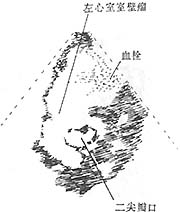
Figure 5 Giant anterior wall left ventricular aneurysm with mural thrombus at the mitral valve level
Short-axis two-dimensional echocardiogram: shows the giant ventricular aneurysm and the thrombus within it
(4) Embolism is caused by the detachment and fragmentation of mural thrombi in the ventricles or deep vein thrombi in the lower limbs. The general incidence abroad is around 10%, while in China, it is usually below 2%. It occurs 1-2 weeks after the onset of the disease. If the embolus originates from the left ventricle, it can lead to cerebral, renal, splenic, or limb arterial embolism; if the embolus comes from the deep veins of the lower limbs, it can result in pulmonary arterial embolism.
(5) Post-myocardial infarction syndrome: Occurs within weeks to months after myocardial infarction, occasionally within days, and may recur. Manifestations include pericarditis, pleuritis, or pneumonia, with symptoms such as fever, chest pain, shortness of breath, and cough, likely caused by an allergic reaction of the body to necrotic material.
(6) Others: Includes infections of the respiratory tract (especially the lungs) or other sites, shoulder-hand syndrome (shoulder stiffness), etc.
(1) Angina Pectoris The nature of pain in angina pectoris is the same as that in myocardial infarction, but the attacks are more frequent, shorter in duration (generally not exceeding 15 minutes), and often preceded by triggering factors. It is not accompanied by fever, leukocytosis, increased erythrocyte sedimentation rate, or elevated serum cardiac enzymes. The electrocardiogram (ECG) may show no changes or only transient ST-segment depression or elevation. Arrhythmias, shock, and heart failure are rare, and the pain is effectively relieved by nitroglycerin, which helps differentiate it from myocardial infarction.
(2) Acute Pericarditis Especially acute nonspecific pericarditis, which may cause severe and prolonged precordial pain with ST-segment and T-wave changes on the ECG. However, patients with pericarditis typically have fever and elevated white blood cell counts either concurrently or prior to the onset of pain. The pain often worsens with deep breathing or coughing, and a pericardial friction rub may be detected on physical examination. The condition is generally less severe than myocardial infarction. On the ECG, ST-segment elevation with a concave upward appearance is seen in all leads except aVR, and no abnormal Q waves are present.
(3) Acute Pulmonary Embolism Massive pulmonary embolism often causes chest pain, dyspnea, and shock, but it is accompanied by signs of acute right heart overload, such as rapid right ventricular enlargement, increased pulsation and accentuated second heart sound over the pulmonary valve area, and a systolic murmur over the tricuspid area. Fever and leukocytosis also appear earlier. The ECG shows right axis deviation, an S wave or deepening of an existing S wave in lead I, a Q wave and T-wave inversion in lead III, a tall R wave in aVR, leftward shift of the transitional zone in the precordial leads, and T-wave inversion in the left precordial leads. These findings differ from those of myocardial infarction and aid in differentiation.
(4) Acute Abdominal Conditions Acute pancreatitis, perforated peptic ulcer, acute cholecystitis, or gallstones may cause upper abdominal pain and shock, which can be confused with myocardial infarction when the pain radiates to the upper abdomen. However, careful history-taking and physical examination can usually distinguish these conditions. ECG and serum cardiac enzyme tests help confirm the diagnosis.
(5) Aortic Dissection Aortic dissection presents with severe chest pain, resembling acute myocardial infarction. However, the pain typically peaks at onset and often radiates to the back, ribs, abdomen, lower back, and lower limbs. There may be significant differences in blood pressure and pulse between the two arms, and some patients may develop aortic regurgitation or transient lower limb paralysis or hemiplegia. Chest X-ray, CT, or echocardiography can detect fluid within the aortic wall, aiding in differentiation. {|104|}
bubble_chart Other Related Items