| disease | Acute Respiratory Distress Syndrome |
| alias | ARDS |
Acute respiratory distress syndrome (ARDS) refers to a clinical syndrome characterized primarily by alveolar-capillary injury following pulmonary or extrapulmonary insults such as severe infection, trauma, or shock. It represents a severe stage or type of acute lung injury (ALI). The clinical features include rapid and labored breathing, progressive hypoxemia, and diffuse alveolar infiltrates on X-ray. This condition closely resembles infant respiratory distress syndrome, but its etiology and pathogenesis differ. To distinguish between them, Ashbauth proposed the term "adult respiratory distress syndrome" in 1972. However, it has since been recognized that this syndrome also occurs in children. Consequently, European and American scholars reached a consensus to replace "adult" with "acute," resulting in the current designation of acute respiratory distress syndrome, still abbreviated as ARDS.
bubble_chart Etiology
The causes of ARDS are numerous, classified by nature as shown in Table 1, with each category encompassing several diseases or causative factors.
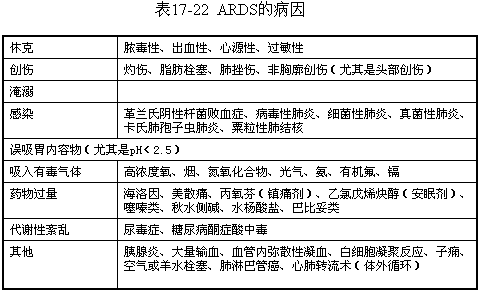
The causes of ARDS vary, but the pathophysiology and clinical course are largely independent of the specific cause. The common foundation is acute injury to the alveolar-capillary membrane. Lung injury can be direct, such as inhalation of gastric acid or toxic gases, chest trauma, etc., leading to physical or chemical injury to the endothelium or epithelial cells. More commonly, however, it is indirect lung injury. Although the mechanism of lung injury has not been fully elucidated, it has been confirmed to be part of the systemic inflammatory response syndrome. At the alveolar-capillary level, the acute inflammatory response mediated by cells and humoral factors involves two main processes: the migration and aggregation of inflammatory cells, and the release of inflammatory mediators. These processes complement each other, acting on specific components of the alveolar-capillary membrane, thereby increasing permeability.
(1) Migration and Aggregation of Inflammatory Cells Almost all lung cells are involved in the pathogenesis of ARDS to varying degrees, with polymorphonuclear neutrophils (PMNs) being one of the most important effector cells in the acute inflammation of ARDS. In normal human lungs, only a small number of PMNs are present in the interstitium, accounting for about 1.6%. In conditions such as trauma, sepsis, acute pancreatitis, physical or chemical stimulation, or extracorporeal circulation, factors like endotoxin lipopolysaccharide (LPS), C5a, and interleukin-8 (IL-8) cause PMNs to accumulate extensively in the pulmonary capillaries. Initially, they adhere to the endothelial cells and then transmigrate across the endothelium into the lung interstitium, eventually moving into the alveolar space via desquamation of the alveolar epithelium. This process involves the participation and regulation of various adhesion molecules. The respiratory burst and release of products by PMNs are critical steps in lung injury. Alveolar macrophages (AMs), in addition to functioning as phagocytes and antigen-presenting cells in immune responses, are also important effector cells in the inflammatory response and contribute to the pathogenesis of ARDS. Stimulated and activated AMs release IL-1, tumor necrosis factor-α (TNF-α), and IL-8, which promote the chemotaxis and aggregation of PMNs in the lungs, likely serving as initiating factors for ALI. Platelet aggregation and microthrombi are common pathological changes in ARDS, and it is speculated that platelets and their products also play a significant role in the disease mechanism of ARDS. Recent studies have found that structural cells such as pulmonary capillary and alveolar epithelial cells are not only target cells but also participate in inflammatory and immune responses, holding special significance in the secondary inflammatory response of ARDS. (II) Release of Inflammatory Mediators The activation of inflammatory cells and the release of mediators are inseparable and coexist with the inflammatory response. They are discussed separately here only for the sake of clarity. Taking bacterial LPS stimulation as an example, it binds to receptors on the surface of macrophages, causing cell shedding and the release of numerous mediators from cellular organelles, including: ① Lipid mediators: Such as metabolites of arachidonic acid and platelet-activating factor (PAF); ② Reactive oxygen metabolites: Including superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radicals (OH·), and singlet oxygen (IO2). Except for H2O2, these oxygen species are self-exaggerating. ③ Peptide substances: Such as PMNs/AMs proteases, complement substrates, various components involved in coagulation and fibrinolysis, cytokines, and even integrins belonging to the adhesion molecule family are sometimes classified as such mediators. In earlier years, much research focused on the first two types of mediators, but in recent years, more attention has been paid to peptide mediators, particularly pro-inflammatory cytokines and adhesion molecules. They may be crucial mediators in initiating and driving the "inflammatory cascade" of ARDS, cell chemotaxis, trans-membrane migration and aggregation, inflammatory responses, and the release of secondary mediators.(3) Alveolar capillary injury and increased permeability. The components that maintain and regulate the structural integrity and permeability of capillaries include the extracellular matrix, intercellular junctions, cytoskeleton, and the interaction between pinocytotic transport and cell substrates. Both direct and indirect injuries in ARDS can affect each of these aspects. Oxygen free radicals, proteases, cytokines, arachidonic acid metabolites, and highly charged products (such as the major cationic proteins of neutrophils) can alter the permeability of the membrane barrier through the following pathways: (1) Cleaving basement membrane proteins and/or cell adhesion factors; (2) Altering the structure of the extracellular fibrous matrix network; (3) Affecting the filament system of the cytoskeleton, leading to cell deformation and junctional tearing.
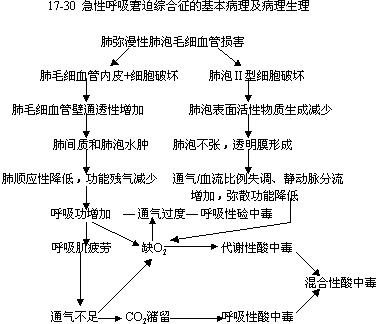
Pathophysiology(1) Basic Pathophysiology. This can be illustrated in Figure 1. It is important to note that ARDS injury and its pathological changes are generally considered diffuse. However, recent studies using imaging and inert gas measurements for gas exchange indicate that lung injury is not as diffuse and uniform as previously understood. Thus, a "two-compartment model" has been proposed: one compartment consists of near-normal lungs that respond normally to applied pressure and ventilation; the other compartment consists of diseased lungs with reduced expansion and ventilation but receiving disproportionately high blood flow. In the early stages, many recruitable lung units in both compartments can interchange with changes in applied pressure or body position, resulting in significant hysteresis and a biphasic shape in the static pressure-volume curve. Early pulmonary edema reduces alveolar volume, which, in a sense, is merely a reduction in air filling rather than a decrease in lung capacity itself. At functional residual capacity, the total lung and thoracic volumes remain within the normal range, and specific compliance (compliance/lung volume) is also normal.
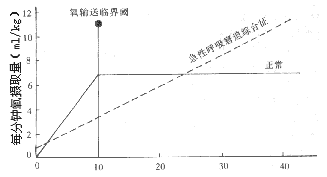
(2) Pathological Dependence of Oxygen Consumption on Oxygen Delivery and Multiple Organ Failure
In recent years, some studies have found abnormalities in the oxygen consumption-oxygen delivery (VO₂/QO₂) relationship in ARDS, which is considered to be the common pathophysiological basis of ARDS and multiple organ failure. In healthy individuals, oxygen delivery can vary, and even if it decreases, the oxygen extraction and consumption by organs remain relatively stable—that is, above a critical threshold, organ oxygen consumption does not depend on oxygen delivery. This is due to local compensatory mechanisms, increased perfusion of capillary cross-sections, and enhanced oxygen extraction. In ARDS, these compensatory mechanisms are exhausted, leading to an absolute or pathological dependence of oxygen consumption on oxygen delivery at all levels of oxygen delivery (Figure 2). This pathological phenomenon manifests as VA/Q mismatch in the lungs and impaired oxygen exchange between tissues and capillaries in extrapulmonary organs. Abnormalities in the VO₂/QO₂ relationship result in cellular hypoxia and metabolic dysfunction, leading to injury. The imbalance between oxygen supply and demand arises from the exhaustion of local compensatory mechanisms. One explanation is the redistribution of blood flow to low-consumption organs such as skeletal muscles, depriving vital organs of adequate oxygen supply. Another explanation is endothelial injury in the capillaries of vital organs, tissue edema, increased diffusion distance, and reduced capillary cross-sectional area. The fundamental cause of injury is the widespread activation of inflammatory cells and the release of mediators. Currently, the latter view is more widely accepted, and it is believed that ARDS and multiple organ failure share a common disease mechanism. Due to the particularly rich capillary network in the lungs, they often become the primary target of inflammatory injury. If early rescue efforts for ARDS are effective or the systemic inflammatory response is self-limited or controlled, the disease course may manifest only as ARDS without progressing to multiple organ failure. However, if ARDS progresses or evolves into multiple organ failure, infection may be the most critical triggering or driving factor.
bubble_chart Pathological Changes
The pathological changes of ARDS caused by various diseases are essentially the same and can be divided into three interrelated and partially overlapping stages: exudation, proliferation, and fibrosis.
(1) Exudative phase: Occurs within the first week of onset. The lungs appear dark red or purplish with hepatization, showing edema and hemorrhage. The weight increases significantly. Microscopic examination within 24 hours reveals pulmonary microvascular congestion, hemorrhage, microthrombi, as well as protein-rich edema fluid and inflammatory cell infiltration in the pulmonary interstitium and alveoli. In cases caused by sexually transmitted infections, the aggregation and infiltration of PMNs in the alveolar spaces are more pronounced. After 72 hours, plasma protein coagulation, cellular fragmentation, and fibrin formation lead to the development of hyaline membranes, with focal or extensive alveolar collapse and atelectasis. During the acute exudative phase, type I pneumocytes are damaged and undergo necrosis.
(2) Proliferative phase: Occurs 1–3 weeks after injury. Type II alveolar epithelial cells proliferate to cover the denuded basement membrane. Fibrosis can be observed in alveolar sacs and ducts, while muscular small stirred pulses exhibit fibrocellular intimal hyperplasia, resulting in a reduction in vascular cross-sectional area.
(3) Fibrotic phase: In ARDS patients surviving beyond 3–4 weeks, the alveolar septa and airspace walls become extensively thickened, with scattered and irregular collagenous connective tissue proliferation leading to diffuse fibrosis. The pulmonary vascular bed undergoes widespread fibrotic thickening of the vessel walls, with stirred pulses becoming deformed and twisted, and pulmonary vessels dilating. Even in ARDS cases not caused by sexually transmitted infections, late-stage (third stage) inevitably involves secondary pulmonary infections, often accompanied by tissue necrosis and microabscesses.
bubble_chart Clinical Manifestations
Apart from the corresponding signs of disease onset, patients may show no respiratory symptoms within the first few hours after lung injury. Subsequently, the respiratory rate increases, and dyspnea gradually worsens. No abnormalities are detected in the lungs, or fine moist rales may be heard during inspiration. Chest X-rays show clear lung fields or only increased and blurred lung markings, suggesting perivascular fluid accumulation. Arterial blood gas analysis reveals low PaO2 and PaO2. As the condition progresses, patients experience respiratory distress, chest tightness, labored inspiration, cyanosis, and often dysphoria and restlessness. Widespread interstitial infiltrates appear in both lungs, possibly accompanied by azygos vein dilation, pleural reaction, or a small amount of effusion. Due to significant hypoxemia-induced hyperventilation, PaCO2 decreases, leading to respiratory alkalosis. The respiratory distress cannot be alleviated by conventional oxygen therapy. If the condition continues to deteriorate as described, respiratory distress and cyanosis worsen further. Chest X-rays show large areas of coalescing lung infiltrates, eventually developing into "white lung." Respiratory muscle fatigue leads to hypoventilation, carbon dioxide retention, and mixed acidosis. Cardiac arrest may occur. Some patients develop multiple organ failure.
bubble_chart Auxiliary Examination
(1) Pulmonary Function Testing
1. Spirometry Measurement Lung volume and vital capacity, residual volume, and functional residual capacity are all reduced. The respiratory dead space increases. If the dead space volume/tidal volume (VD/VT) ratio exceeds 0.6, mechanical ventilation is indicated.
2. Lung Compliance Measurement: At the bedside, total thoracic-pulmonary compliance is commonly measured. For patients on positive end-expiratory pressure (PEEP) ventilation, dynamic compliance (Cdyn) can be calculated using the following formula. Compliance testing is not only valuable for diagnosis and evaluating treatment efficacy but also for monitoring complications such as pneumothorax or atelectasis.
| Cdyn | = | Tidal Volume |
|
Maximum Airway Pressure - Positive End-Expiratory Pressure |
3. Arterial Blood Gas Analysis PaO2 reduction is a common indicator for ARDS diagnosis and monitoring. Based on arterial blood gas analysis, derived indices such as the alveolar-arterial oxygen gradient (PA-aO2), venous admixture (Qs/Qt), respiratory index (PA-aO2/PaO2), and oxygenation index (PaO2/FiO2) can be calculated, which are highly useful for diagnosis and assessing disease severity. For example, Qs/Qt is recommended for disease grading, with values above 15%, 25%, and 35% indicating mild, moderate, and severe disease, respectively. The reference range for the respiratory index is 0.1–0.37; a value >1 indicates significant impairment in oxygenation, and >2 often necessitates mechanical ventilation. The oxygenation index reference range is 53.2–66.7 kPa (400–500 mmHg), which drops to 26.7 kPa (20 mmHg) in ARDS.
(2) Pulmonary Vascular Permeability and Hemodynamic Measurements
1. Pulmonary Edema Fluid Protein Measurement In ARDS, increased pulmonary capillary permeability allows water and large-molecular proteins to enter the interstitium or alveoli, raising the ratio of edema fluid protein to plasma protein. A ratio >0.7 suggests ARDS, while <0.5 indicates cardiogenic pulmonary edema.
2. Alveolar-Capillary Membrane Permeability (ACMP) Measurement Using dual-nuclide in vivo labeling techniques, autologous transferrin is labeled with 113indium (113In) to measure pulmonary protein accumulation, while autologous red blood cells are labeled with 99mtechnetium (99mTc) to correct for intrathoracic blood flow distribution. The lung-to-heart radioactivity ratios for 113indium and 99mtechnetium are calculated separately, and changes over 2 hours yield the plasma protein accumulation index. The healthy reference value is 0.138 × 10-3/min.
3. Hemodynamic Monitoring By inserting a four-lumen floating catheter, pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), pulmonary vascular resistance (PVR), PVO2, CVO2, Qs/Qt, and cardiac output (CO) measured by thermal dilution can be simultaneously determined and calculated. This is not only valuable for diagnosis and differential diagnosis but also serves as an important monitoring indicator for mechanical ventilation therapy, especially the impact of PEEP on circulatory function. In ARDS patients, the mean pulmonary artery pressure is significantly elevated (>2.67 kPa), the pulmonary artery pressure-pulmonary capillary wedge pressure gradient (PAP-PCWP) increases (>0.67 kPa), and PCWP is generally <1.18 kPa (12 cmH2O). If PCWP exceeds 1.57 kPa (16 cmH2O), it indicates acute left heart failure and can exclude ARDS.
4. Measurement of Extravascular Lung Water Currently, the double-indicator dilution technique is used for measurement. A 5 cm indocyanine green dye glucose solution (10 ml) is injected via a central venous or right heart catheter. Then, a thermodilution curve is recorded through a femoral artery catheter connected to a thermistor, while a densitometer detects the dye dilution curve. The data is processed by a computer to calculate lung water content, which can be used to assess the degree of pulmonary edema, its progression, and treatment efficacy. However, this method requires specific equipment.
To date, the lack of specific detection indicators has made early diagnosis difficult. For any underlying disease or predisposing factor that may cause ARDS, once respiratory changes or abnormal blood gas levels are observed, the possibility of this syndrome should be alerted. The diagnosis is established by integrating clinical, laboratory, and auxiliary examinations, along with necessary dynamic follow-up observations, while excluding other diseases with similar manifestations. For disease statistics and research purposes, a definitive diagnostic standard must be followed. Over the years, various diagnostic criteria have been proposed by different experts, with significant differences. The definitions and diagnostic criteria for ALI and ARDS, discussed by European and American scholars at academic conferences in the United States and Europe in 1992, jointly proposed in 1992, and published in various journals in 1994, have recently been widely introduced and recommended in China.
ARDS Diagnostic Criteria
Except for the requirement of PaO₂/FiO₂ ≤ 26.7 kPa (200 mmHg), the remaining indicators are the same as those for ALI.
In 1995, the National Academic Conference on Critical Care Medicine (Lushan) proposed China's staged diagnostic criteria for ARDS based on the above standards as follows:
1. Presence of an underlying disease or cause that may induce ARDS.
2. Diagnosis of the premonitory stage of ARDS requires meeting three of the following five criteria:
⑴ Respiratory rate of 20–25 breaths per minute.
⑵ (FiO20.21) PaO2 ≤ 9.31 kPa (≤70 mmHg), >7.8 kPa (60 mmHg).
⑶ PaO2/FiO2 ≥ 39.9 kPa (≥300 mmHg).
⑷ PA-aO2(FiO20.21) 3.32–6.65 kPa (25–50 mmHg).
⑸ Normal chest X-ray.
3. Diagnosis of early-stage ARDS requires meeting three of the following six criteria:
⑴ Respiratory rate >28 breaths per minute.
⑵ (FiO20.21) PaO2 ≤7.90 kPa (60 mmHg) >6.60 kPa (50 mmHg).
⑶ PaCO2 <4.65 kPa (35 mmHg).
⑷ PaO2/FiO2 ≤39.90 kPa (≤300 mmHg) >26.60 kPa (>200 mmHg).
⑸ (FiO21.0) PA-aO2 >13.30 kPa (>100 mmHg) <26.60 kPa (<200 mmHg).
⑹ Chest X-ray shows no alveolar consolidation or consolidation ≤1/2 of the lung field.
4. Diagnosis of advanced-stage ARDS requires meeting three of the following six criteria:
⑴ Respiratory distress, rate >28 breaths per minute.
⑵ (FiO20.21) PaO2 ≤6.60 kPa (≤50 mmHg).
⑶ PaCO2 >5.98 kPa (>45 mmHg).
⑷ PaO2/FiO2 ≤26.6 kPa (≤200 mmHg).
⑸(FiO21. 0)PA-aO2>26. 6kPa(>200mmHg)
⑹Chest X-ray shows alveolar consolidation involving ≥1/2 of the lung field.
bubble_chart Treatment Measures
The key to ARDS treatment lies in addressing the primary disease and its {|###|}disease cause{|###|}. For instance, managing trauma, identifying the infection site in a timely manner, and using sensitive antibiotics to target the pathogenic bacteria can halt further inflammatory damage to the lungs. More urgently, it is crucial to promptly correct the patient's severe hypoxia to gain valuable time for treating the underlying condition. During respiratory support therapy, complications such as barotrauma, secondary respiratory infections, and oxygen toxicity must be prevented. Exploring new pharmacological treatments based on the {|###|}mechanism of disease{|###|} in lung {|###|}injury{|###|} is also an important research direction.
(1) Respiratory Support Therapy
1. Oxygen Therapy: Correcting hypoxia is an urgent priority. Continuous positive airway pressure (CPAP) via a facemask can be used, but most cases require mechanical ventilation for oxygen delivery. Generally, when FiO2 > 0.6, PaO2 remains < 8 kPa (60 mmHg), and SaO2 < 90%, comprehensive treatment centered on positive end-expiratory pressure (PEEP) should be initiated.
2. Mechanical Ventilation
(1) Positive End-Expiratory Pressure (PEEP): In 1969, Ashbaugh first reported using PEEP to treat 5 ARDS patients, with 3 surviving. After years of clinical practice, PEEP has become a critical measure for rescuing ARDS patients. PEEP improves respiratory function in ARDS primarily by applying positive pressure at the end of inspiration to reopen collapsed bronchi and alveoli, thereby increasing functional residual capacity (FRC).
When PEEP is 0.49 kPa (5 cmH2O), FRC can increase by 500 ml. As the collapsed alveoli reopen, the pulmonary venous-arterial blood flow decreases, the ventilation/perfusion ratio and diffusion function improve, and it has a beneficial effect on pulmonary extravascular edema, enhancing lung compliance and reducing the work of breathing. PaO2 and SaO2 continuously improve with increasing PEEP. If cardiac output remains unaffected, systemic oxygen delivery increases. Animal experiments have shown that as PEEP increases from zero to 0.98 kPa (10 cmH2O), alveolar diameter increases proportionally, while intrathoracic pressure changes little. When PEEP > 0.98 kPa, the change in alveolar diameter becomes smaller. At PEEP > 1.47 kPa (15 cmH2O), alveolar volume increases minimally, and instead, intrathoracic pressure rises with increasing alveolar pressure, affecting venous return. Especially in cases of insufficient blood volume or poor vasoconstrictive regulation, this can reduce cardiac output. Therefore, although excessively high PEEP may increase PaO2 and SaO2, it often reduces tissue oxygen supply due to decreased cardiac output. Excessively high PEEP also increases the incidence of pneumothorax and mediastinal emphysema. The optimal PEEP should achieve SaO2 above 90% while reducing FiO2 to a safe level [generally 1.47 kPa (15 cmH2O)]. For patients maintaining effective blood volume and ensuring tissue perfusion, PEEP should start at a low level of 0.29–0.49 kPa (3–5 cmH2O) and gradually increase to the optimal PEEP. If PEEP > 1.47 kPa (15 cmH2O) and SaO2 < 90%, FiO2 may be temporarily increased (preferably not exceeding 6 hours) to achieve SaO2 above 90%. Further investigation should be conducted to identify and address the underlying causes of refractory hypoxemia. Once the condition stabilizes, FiO2 should be gradually reduced to below 50%, followed by lowering PEEP to ≤0.49 kPa (5 cmH2O) to consolidate therapeutic effects.
(2) Inverse Ratio Ventilation (IRV) This refers to a mechanical ventilation mode where the inspiratory (I) to expiratory (E) time ratio is ≥1:1. Prolonging the positive-pressure inspiratory time facilitates gas entry into alveoli with longer time constants due to obstruction, promoting their recruitment and restoring ventilation. It also redistributes ventilation from rapidly inflating alveoli to slower ones, improving gas distribution, the ventilation-perfusion ratio, and increasing the diffusion surface area. Shortening the expiratory time keeps alveolar volume above the closing volume of small airways, mimicking the effect of PEEP. IRV can reduce peak airway pressure and PEEP while increasing mean airway pressure (MAP), leading to an increase in PaO2/FiO2 as MAP rises. Similarly, extending the end-inspiratory pause time enhances hemoglobin oxygenation. Therefore, IRV can be attempted in ARDS patients with poor response to PEEP. However, excessive MAP may still cause barotrauma, impair circulatory function, and reduce cardiac output. Hence, MAP should ideally not exceed 1.37 kPa (14 cmH2O). Patients may experience discomfort during IRV, and sedation or anesthesia may be required.
(3) Prevention and Management of Mechanical Ventilation Complications The most common and life-threatening complication of mechanical ventilation itself is barotrauma. Due to widespread inflammation, congestion, edema, and alveolar collapse in ARDS, mechanical ventilation often requires higher peak inspiratory pressures. Combined with high levels of PEEP, increased MAP can lead to overdistension of less affected, more compliant lung units, resulting in alveolar rupture. Reports indicate that when PEEP > 2.45 kPa (25 cmH2O), the incidence of pneumothorax and mediastinal emphysema reaches 14%, with a mortality rate approaching 100%. Some scholars now advocate for low tidal volume and low ventilation, even permitting mild hypoventilation and grade I hypercapnia, to maintain peak inspiratory pressure (PIP) < 3.92 kPa (40 cmH2O) and PEEP < 1.47 kPa (15 cmH2O). Pressure-regulated volume control (PRVCV) or pressure-controlled inverse ratio ventilation (PIP < 2.94–3.43 kPa [30–35 cmH2O]) may be used if necessary. Other approaches, such as inhaled nitric oxide (NO), extracorporeal membrane oxygenation (ECMO), or high-frequency ventilation, can reduce or prevent barotrauma from mechanical ventilation.
3. Membrane Oxygenator In cases where ARDS patients show poor response to mechanical ventilation via an artificial airway and oxygen therapy, and respiratory function cannot be corrected in the short term, extracorporeal membrane oxygenation (ECMO) has been employed. This involves inserting catheters into the inferior vena cava via the bilateral great saphenous veins. The development of intravascular oxygenator/CO2 removal devices (IVOX) has enabled hollow-fiber membranes with oxygenation and CO2 removal capabilities to be inserted via the femoral vein into the inferior vena cava. A negative pressure draws nitrogen through IVOX, improving gas exchange. Combined with mechanical ventilation, this can reduce certain ventilation parameters and minimize complications.
(II) Maintaining appropriate blood volume Excessive bleeding from trauma necessitates blood transfusion. Avoid over-transfusion, and the drip rate should not be too fast. Fresh blood is preferable. Blood stored for more than one week contains micro-particles that can cause micro-embolisms and damage pulmonary capillary endothelial cells, so a micro-filter must be used. Under the premise of ensuring blood volume and stabilizing blood pressure, a Grade I negative fluid balance (-500 to -1000ml/day) is recommended. To promote the resolution of edema, furosemide (Lasix) can be administered at 40–60mg daily. When endothelial cell permeability increases, colloids can leak into the interstitial space, worsening pulmonary edema. Therefore, colloid solutions should not be administered in the early stages of ARDS, unless serum protein levels are low.
(3) Application of Adrenocortical Hormones It protects capillary endothelial cells, prevents the aggregation and adhesion of white blood cells and platelets to the vessel walls to form microthrombi; stabilizes lysosomal membranes, reduces complement activity, inhibits phospholipid metabolism on cell membranes, decreases the synthesis of arachidonic acid, and blocks the production of prostaglandins and thromboxane A2; protects type II alveolar cells from secreting surfactant; has anti-inflammatory effects and promotes the absorption of interstitial lung fluid; relieves bronchospasm; and inhibits late-stage [third stage] pulmonary fibrosis. Currently, it is believed that hormones can be used early for ARDS caused by non-infectious factors such as inhalation of irritant gases or traumatic fractures leading to fat embolism. Dexamethasone 60–80 mg/d or hydrocortisone 1000–2000 mg/d, administered every 6 hours for 2 days, may be continued for 1–2 more days if effective, but discontinued early if ineffective. Hormones are contraindicated in ARDS accompanied by sepsis or severe respiratory infections.
(4) Correction of Acid-Base and Electrolyte Imbalances The general principles are the same as for respiratory failure, with an emphasis on prevention.
(5) Nutritional Support ARDS patients are in a hypermetabolic state and should promptly receive supplemental calories and high-protein, high-fat nutrients. Strong nutritional support should be provided as early as possible, either via nasogastric feeding or intravenous supplementation, maintaining a total caloric intake of 83.7–167.4 kJ (20–40 kcal/kg).
(6) Other Investigational Therapies
1. Surfactant Replacement Therapy Currently, both natural extracts and synthetic surfactants are available domestically and internationally, showing good efficacy in treating infant respiratory distress syndrome. However, exogenous surfactants in ARDS only temporarily increase PaO2.
2. Inhaled NO NO, or endothelial-derived relaxing factor, has broad physiological activity and is involved in the pathophysiological processes of many diseases. Its physiological role and potential clinical applications in ARDS have been extensively studied. Generally, NO enters well-ventilated lung regions, dilates the pulmonary vasculature in those areas, redirecting blood flow from poorly ventilated regions to the dilated vessels, improving ventilation-perfusion ratios, reducing intrapulmonary shunting, and thereby lowering the required oxygen concentration. Additionally, NO reduces pulmonary artery pressure and pulmonary vascular resistance without affecting systemic vasodilation or cardiac output. Some researchers report that combining inhaled NO with intravenous almitrine bismesylate has a synergistic effect on improving gas exchange and reducing mean pulmonary artery pressure. The latter constricts blood vessels in poorly ventilated lung regions, redirecting blood flow to better-ventilated areas, and stimulates peripheral chemoreceptors to enhance respiratory drive and increase ventilation. The potential increase in pulmonary artery pressure caused by almitrine may be offset by NO. However, the clinical application of NO requires further in-depth study, and many practical issues remain to be resolved.
3. Oxygen Free Radical Scavengers, Antioxidants, and Immunotherapy Based on the pathogenesis of ARDS, targeting key disease mechanisms to develop corresponding interventions and reduce lung and other organ damage is currently a major research focus.
Superoxide dismutase (SOD) and catalase (CAT) can prevent acute lung injury caused by the oxidative effects of O2 and H2O2. Uric acid inhibits the production of O2 and OH, as well as PMN respiratory bursts. Vitamin E has some antioxidant properties but may increase the risk of nosocomial infections.
Inhibitors of the lipoxygenase and cyclooxygenase pathways, such as ibuprofen, can reduce thromboxane A2 and prostaglandins, inhibit the binding of complement to PMNs, and prevent PMN aggregation in the lungs.
Immunotherapy treats ARDS by neutralizing disease-causing factors, combating inflammatory mediators, and inhibiting effector cells. Currently, extensively researched approaches include anti-endotoxin antibodies, anti-TNF, IL-1, IL-6, IL-8, as well as antibodies or drugs targeting cell adhesion molecules.
The prognosis of ARDS is not only related to whether the rescue measures are appropriate but also often associated with the patient's primary disease, complications, and response to treatment. For example, if severe infection-induced sepsis is not controlled, the prognosis is extremely poor. The mortality rate of ARDS complicating bone marrow transplantation is almost 100%. If multiple organ failure occurs, the prognosis is extremely poor and related to the number and speed of affected organs. For instance, if three organ failures persist for more than one week, the mortality rate can be as high as 98%. After active treatment, if pulmonary vascular resistance continues to increase, it indicates a poor prognosis. For ARDS caused by fat embolism, with aggressive management and mechanical ventilation, a 90% survival rate can be achieved. Acute pulmonary edema and ARDS caused by irritant gases generally have better outcomes if the patient is removed from the exposure site and treated promptly. Additionally, if ARDS patients show a significant increase in PaO2 after PEEP0.98 (10cmH2O) treatment, the prognosis is relatively favorable. Most patients whose ARDS symptoms are rapidly alleviated can recover fully. Among the 40% of ARDS survivors with abnormal lung function, 20% exhibit obstructive ventilation impairment, 30% have reduced diffusion capacity, and 25% show a decrease in PaO2 during exercise.
High-risk patients should be closely monitored and given intensive care. Once manifestations of lung injury such as rapid respiration and decreased PaO2 are observed, respiratory support and other effective preventive and interventional measures should be administered early while treating the primary condition, to prevent the progression of ARDS and injury to vital organs.




