| disease | Acute Pericarditis |
| alias | Caute Pericarditis |
Pericarditis is the most common pericardial disorder, which can be caused by various disease factors and is often part of systemic diseases or spreads from adjacent tissue lesions. Pericarditis can coexist with inflammation of other cardiac structures such as the myocardium or endocardium, or it can exist alone. Pericarditis can be divided into acute and chronic types, with the former often accompanied by pericardial effusion and the latter often leading to pericardial constriction. Acute pericarditis is an acute inflammation of the visceral and parietal layers of the pericardial membrane, which can be accompanied by myocarditis and endocarditis, or it can appear as the sole cardiac lesion.
bubble_chart Etiology
Acute pericarditis is almost always secondary, and its disease cause is essentially various primary medical and surgical diseases, with some disease causes still unknown. Among them, non-specific, subcutaneous node, purulent, and wind-dampness pericarditis are relatively common. Foreign data indicate that non-specific pericarditis has become the main type of pericarditis in adults; domestic reports show that subcutaneous node pericarditis is the most common, followed by non-specific pericarditis. Pericarditis caused by malignant tumors and acute myocardial infarction is on the rise. With the advancement of antibiotics and chemotherapy, the incidence of subcutaneous node, purulent, and wind-dampness pericarditis has significantly decreased. Except for systemic lupus erythematosus pericarditis, the incidence rate is significantly higher in males than in females.
Classification of disease causes of acute pericarditis
(1) Non-specific pericarditis
(2) Infectious pericarditis1. Bacterial ⑴ Purulent, ⑵ Subcutaneous node;
2. Viral such as Coxsackie, Echo, influenza, pestilence mononucleosis, and cytomegalovirus, etc.;
3. Fungal such as Histoplasma, Actinomyces, Nocardia, Aspergillus, Mycobacterium, etc.;
4. Others such as Rickettsia, Spirochete, Mycoplasma, Paragonimus, Amoeba, and Hydatid, etc.
(3) Pericarditis associated with other organ or tissue system diseases
1. Autoimmune diseases such as wind-dampness heat, rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis, scleroderma, polyarthritis, post-pericardiotomy syndrome, post-myocardial infarction syndrome, dialysis treatment, kidney transplantation, and Acquired Immune Deficiency Syndrome, etc.;
2. Allergic diseases such as serum sickness, allergic granulomatosis, and allergic pneumonia, etc.;
3. Diseases of adjacent organs such as myocardial infarction, dissecting stirred pulse tumor, pulmonary embolism, pleural membrane, lung and esophageal diseases, etc.;
4. Endocrine and metabolic diseases such as uremia, myxedema, diabetes, pain wind, Addison's disease, cholesterol pericarditis, etc.;
5. Others such as pancreatitis, thalassemia, intestinal fat metabolism disorder, non-gonococcal arthritis, conjunctival membrane, urethritis syndrome, etc.
(4) Pericarditis caused by physical factors
1. Trauma such as penetrating injuries, foreign bodies, cardiac catheters, artificial pacemakers, and cardiac tuina, etc.;
2. Radiation.
(5) Drug-induced pericarditis such as hydralazine, procainamide, phenytoin sodium, penicillin, isoniazid, phenylbutazone, and methylthiouracil, etc.
(6) Neoplastic pericarditis
1. Primary mesothelioma, fleshy tumor, etc.;
2. Secondary metastasis from lung or breast cancer, multiple myeloma, leukemia, and lymphoma, etc.Pericardial effusion is the primary cause of a series of pathophysiological changes induced by acute pericarditis. Due to gravity, pericardial effusion initially accumulates at the diaphragmatic surface of the heart. As the effusion increases, it fills the retrosternal pericardial space, and then, except for the posterior part of the atria where the pericardium is reflected, both sides of the heart can be filled with effusion. Due to the rapid or massive accumulation of effusion, the pressure within the pericardial cavity rises. When it reaches a certain level, it restricts the expansion of the heart, reduces ventricular diastolic filling, and decreases stroke volume. At this point, the body's compensatory mechanisms increase venous pressure to enhance ventricular filling, strengthen myocardial contractility to improve ejection fraction, accelerate heart rate to increase cardiac output, and elevate peripheral small artery resistance to maintain arterial blood pressure, thereby maintaining a relatively normal resting cardiac output. If pericardial effusion continues to increase and the pressure within the pericardial cavity further rises, leading to a critical drop in stroke volume, the compensatory mechanisms fail. The elevated venous pressure can no longer increase ventricular filling, the ejection fraction decreases, an excessively rapid heart rate shortens the diastolic period and reduces filling, no longer increasing the minute cardiac output, and small artery contraction reaches its limit, causing arterial blood pressure to drop, leading to a significant decrease in cardiac output, circulatory failure, and shock. This condition is known as cardiac tamponade or pericardial tamponade.
In normal individuals, arterial blood pressure may drop by grade I during inspiration (a decrease not exceeding 1.33 kPa (10 mmHg)), so the intensity of the peripheral pulse does not change significantly. When pericardial effusion causes pericardial tamponade, the pulse intensity may significantly weaken or disappear during inspiration. The mechanisms are: ① During inspiration, the negative intrathoracic pressure significantly increases pulmonary vascular capacity, storing blood within the pulmonary vessels, while the heart, restricted by the surrounding effusion, cannot significantly increase right ventricular filling, and the right ventricular output is insufficient to compensate for the increase in pulmonary blood volume, reducing or even reversing pulmonary venous return, thereby decreasing left ventricular filling; ② The volume of the heart surrounded by fluid is fixed, and during inspiration, the increased filling of the right ventricle enlarges its volume, shifting the interventricular septum backward and reducing left ventricular volume, thus decreasing filling; ③ During inspiration, the descent of the diaphragm stretches the tense pericardium, further increasing the pressure within the pericardial cavity and reducing left ventricular filling. The combination of these three factors sharply reduces left ventricular output, significantly lowering arterial blood pressure [by more than 1.33 kPa (10 mmHg)], resulting in pulsus paradoxus.bubble_chart Pathological Changes
The extent and characteristics of the inflammatory response in pericarditis vary with the disease cause. It can be localized or diffuse, with pathological changes being either fibrinous (dry) or exudative (wet), with the former potentially developing into the latter. The exudate can be serofibrinous, serosanguineous, hemorrhagic, or purulent. At the onset of inflammation, the parietal and visceral pericardium exhibit exudates composed of fibrin, leukocytes, and endothelial cells. As the fluid in the exudate increases, it becomes serofibrinous, with volumes reaching 2-3 liters, appearing straw-yellow and clear, or turbid due to a higher content of leukocytes and endothelial cells; if it contains more red blood cells, it becomes serosanguineous. The exudate is usually absorbed within 2-3 weeks. Subcutaneous node pericarditis often produces a large amount of serofibrinous or serosanguineous exudate, which can persist for months, occasionally presenting as a localized abdominal mass. The exudate in purulent pericarditis contains a large number of neutrophils, appearing as thick pus. In cholesterol pericarditis, the exudate contains a large amount of cholesterol, appearing golden yellow. The exudate in chylous pericarditis appears milky. Hemorrhagic pericarditis caused by subcutaneous nodes or neoplasms contains a large number of red blood cells and should be differentiated from hemopericardium containing pure blood due to trauma or anticoagulant use. The inflammatory response often involves the subepicardial myocardium, and in severe cases, it can extend to the deep myocardium, even spreading to the mediastinum, diaphragm, and pleura. After healing, pericarditis may leave localized small patches, generalized pericardial thickening, or varying degrees of adhesions. Adhesions can completely obliterate the pericardial cavity. If the inflammation involves the outer surface of the pericardial wall, it can cause adhesions between the heart and adjacent tissues (such as the pleura, mediastinum, and diaphragm). The inflammatory exudate in acute fibrinous pericarditis can completely dissolve and be absorbed, persist for a longer period, or organize, being replaced by connective tissue to form scars, even leading to pericardial calcification, eventually developing into constrictive pericarditis.
bubble_chart Clinical Manifestations
(1) Symptoms: May be masked by symptoms of primary diseases such as chills, fever, sweating, lack of strength, etc., during infections. Symptoms of pericarditis itself include:
1. Precordial pain: Mainly seen in the fibrinous exudation stage of inflammatory changes. The visceral and parietal layers of the pericardium have no pain-sensing nerves. The outer surface of the parietal layer below the level of the fifth or sixth intercostal space has pain-sensing fibers of the phrenic nerve. Therefore, pain occurs when the lesion spreads to this part of the pericardium or nearby pleura, mediastinum, or diaphragm. Precordial pain is often exacerbated by changes in body position, deep breathing, coughing, swallowing, lying down, especially when lifting the legs or lying on the left side, and relieved when sitting or leaning forward. The pain is usually localized under the sternum or in the precordial area, often radiating to the left shoulder, back, neck, or upper abdomen, and occasionally to the jaw, left forearm, and hand. Pain at the right trapezius ridge is a specific symptom of pericarditis but is uncommon. Some pericarditis pains are more pronounced, such as acute nonspecific pericarditis; others are mild or completely painless, such as subcutaneous nodular and uremic pericarditis.
2. Symptoms of cardiac tamponade: May include dyspnea, pale complexion, dysphoria, cyanosis, lack of strength, upper abdominal pain, edema, and even shock.
3. Symptoms of pericardial effusion compressing adjacent organs: Compression of the lungs, trachea, bronchi, and large vessels causes pulmonary congestion, reduced lung capacity, restricted ventilation, exacerbating dyspnea, making breathing shallow and rapid. Patients often automatically adopt a forward-leaning sitting position to shift the pericardial effusion downward and forward to relieve compression symptoms. Tracheal compression can cause coughing and hoarseness. Esophageal compression can cause dysphagia.
4. Systemic symptoms: Pericarditis itself can also cause chills, fever, palpitations, sweating, lack of strength, etc., which are often difficult to distinguish from symptoms of the primary disease.
(2) Signs
1. Pericardial friction rub: A typical sign of acute fibrinous pericarditis. The sound produced by the friction between the inflamed and roughened parietal and visceral layers of the pericardium during heart activity, presenting as a scratching, rough, high-frequency sound; often overrides heart sounds and feels closer to the ear than heart sounds. A typical friction rub can be heard with three components corresponding to atrial contraction, ventricular contraction, and ventricular relaxation. Most often, there are two components related to ventricular contraction and relaxation, presenting as a back-and-forth sound. In the initial and final stages of this sound, it may only be heard during ventricular contraction. It can be heard throughout the precordial area but is most distinct at the third and fourth intercostal spaces on the left sternal border, the lower sternum, and near the xiphoid process. Its intensity is often influenced by breathing and body position, increasing with deep inspiration, leaning forward, or having the patient lie prone and pressing the stethoscope firmly against the chest wall. It often appears for only a few hours or persists for days or weeks. When effusion separates the two layers of the pericardium completely, the pericardial friction rub disappears; if the two layers are partially adhered, the friction rub may still be heard despite a large pericardial effusion. Hearing a pericardial friction rub in the precordial area can diagnose pericarditis.
2. Pericardial effusion: Effusion of more than 200-300ml or rapid abdominal mass can produce the following signs:
⑴ Cardiac signs: Weakening or disappearance of the apical impulse or its appearance inside the left border of cardiac dullness. The cardiac dullness boundary expands to both sides, the relative dullness area disappears, and the cardiac dullness boundary at the second and third intercostal spaces widens when the patient changes from sitting to lying down. Heart sounds are faint and distant, and the heart rate is fast. A few patients may hear an early diastolic extra sound (pericardial knock) at the third and fourth intercostal spaces on the left sternal border. This sound occurs about 0.1 seconds after the second heart sound, is loud, and presents as a snapping sound, caused by the sudden cessation of blood flow due to the restriction of ventricular relaxation by pericardial effusion, forming vortices and impacting the ventricular wall to produce vibrations.
(2) Signs of left lung compression: When there is a large amount of pericardial effusion, the heart shifts backward, compressing the left lung, which can lead to atelectasis of the lower lobe of the left lung. A dull area is often found below the left scapula, with increased vocal fremitus, and bronchial breath sounds can be heard (Ewart's sign).
⑶ Signs of cardiac tamponade: Rapid pericardial effusion, even as little as 100ml, can cause acute cardiac tamponade, manifesting as significant tachycardia. If cardiac output significantly decreases, shock may occur. When the effusion accumulates slowly, in addition to increased heart rate, venous pressure significantly rises, leading to distended neck veins, pulsation and expansion during inspiration, hepatomegaly with tenderness, ascites, subcutaneous edema, and positive hepatojugular reflux, indicating systemic venous congestion. The pulse pressure decreases, the pulse becomes weak and thready, and paradoxical pulse may appear.
bubble_chart Auxiliary Examination
Electrocardiogram Examination In acute pericarditis, due to the involvement of the myocardium beneath the visceral pericardium and the effects of pericardial effusion, the following changes may appear on the electrocardiogram:
1. ST segment displacement Due to inflammation and compression of the myocardium beneath the epicardium by pericardial effusion, causing injury and ischemia.
2. T wave changes Due to delayed repolarization of the myocardial fibers beneath the epicardium.
3. Evolution of the electrocardiogram in acute pericarditis The typical evolution can be divided into four stages: ①ST segment elevation with a downward convexity, and high T waves. Generally, acute pericarditis is a diffuse sexually transmitted disease, so it appears in all leads except aVR and V1, lasting from 2 days to about 2 weeks. The ST/T ratio in V6 is ≥0.25. ②A few days later, the ST segment returns to the baseline, and the T wave decreases and flattens. ③The T wave becomes symmetrically inverted and reaches its maximum depth, without corresponding lead antagonism changes (except for aVR and V1, which remain upright). This can last for weeks, months, or long-term. ④The T wave returns to upright, usually within 3 months. In cases of milder or localized disease, atypical evolution may occur, with changes in the ST segment and T wave in some leads, or only changes in the ST segment or T wave.
4. PR segment displacement Except for aVR and V1 leads, the PR segment is depressed, indicating damage to the atrial myocardium beneath the pericardium.
5. Low voltage QRS complex Presumably due to the electrical short-circuiting effect of pericardial effusion. If low voltage persists after removing the pericardial effusion, it should be considered related to the insulating effect of pericardial inflammatory fibrin and surrounding tissue edema.
6. Electrical alternans Total electrical alternans of P, QRS, and T waves is a characteristic electrocardiographic manifestation of large pericardial effusion. The heart tends to swing in a spiral motion during contraction, which is normally limited by the pericardium. When there is a large pericardial effusion, the heart appears to float in the fluid, and the swing amplitude significantly increases. If the heart moves in a regular "counterclockwise rotation-then return" motion at half the heart rate frequency, it causes alternating changes in the electrical axis of the heart.
7. Arrhythmias Sinus tachycardia is common, and some may develop atrial arrhythmias, such as atrial premature beats, atrial tachycardia, atrial flutter, or atrial fibrillation. In wind-dampness pericarditis, varying degrees of atrioventricular block may occur.
(5) X-ray examination When pericardial effusion exceeds 250ml, the cardiac shadow may enlarge, the right cardiophrenic angle becomes sharp, the normal contour of the heart border disappears, appearing as a water droplet or flask shape, and the cardiac shadow moves with changes in body position. Fluoroscopy or X-ray kymography can show weakened or absent cardiac pulsations. X-ray films showing a significantly enlarged cardiac shadow with clear lung fields, or rapid enlargement of the cardiac shadow in a short period on several X-ray films, often provide early and reliable clues for diagnosing pericardial effusion.
Additionally, during right heart catheterization, pushing the catheter against the right atrial border, selective heart blood vessel angiography, or having the patient lie on their left side and injecting 50-100ml of carbon dioxide intravenously before taking an X-ray film, if the distance between the right atrial border endocardial surface and the lung field exceeds 5mm, it has diagnostic value for pericardial effusion.
(6) Ultrasound examination Normally, there may be 20-30ml of lubricating fluid in the pericardial cavity, which is often difficult to detect on echocardiography. If there is a posterior fluid dark area throughout the cardiac cycle, there is at least 50ml of fluid in the pericardial cavity, confirming pericardial effusion. End-diastolic right atrial collapse (Figure 2) and diastolic right ventricular free wall collapse are the most sensitive and specific signs for diagnosing cardiac tamponade. It can be performed at the bedside and is a simple, safe, sensitive, and accurate non-invasive method for diagnosing pericardial effusion.
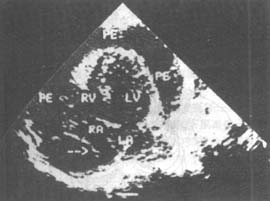
Figure 2 Two-dimensional echocardiogram of pericardial effusion with cardiac tamponade in the apical four-chamber view, arrows indicate diastolic collapse of the right atrial lateral wall.
PE: Pericardial effusion; RA: Right atrium; RV: Right ventricle; LA: Left atrium; LV: Left ventricle.
(7) Radionuclide examination: After intravenous injection of 113mIn or 99mTc, a cardiac blood pool scan is performed. In the presence of pericardial effusion, a blank area around the cardiac chambers is shown, the heart may be reduced in size or normal, the outer edge of the heart is irregular (especially the right edge), and the ratio of the transverse diameter of the scanned heart shadow to the X-ray heart shadow is less than 0.75.
(8) Magnetic resonance imaging: It can clearly show the volume and distribution of pericardial effusion and can distinguish the nature of the effusion, such as non-hemorrhagic effusion which mostly shows low signal intensity; in uremia, trauma, and subcutaneous node effusion, which contains more protein and cells, medium or high signal intensity can be seen.
(9) Pericardiocentesis: In the presence of pericardial effusion, pericardiocentesis can be performed to smear, culture, and search for pathological cells in the effusion, which helps to determine the pathogen. The determination of adenosine deaminase (ADA) activity in pericardial fluid ≧30u/L is highly specific for the diagnosis of subcutaneous node pericarditis. After fluid extraction, air (100-150ml) is injected for X-ray imaging to understand the thickness of the pericardium, whether the pericardial surface is regular (tumors can cause localized bulging), and the size and shape of the heart.
(10) Pericardioscopy: For those with pericardial effusion requiring surgical drainage, pericardioscopy can be performed first. It allows direct visualization of the pericardium and pericardial biopsy in suspicious areas, thereby improving the accuracy of disease cause diagnosis.
The presence of a pericardial friction rub in the precordial area confirms the diagnosis of pericarditis. In diseases that may complicate pericarditis, if symptoms such as chest pain, dyspnea, tachycardia, unexplained systemic venous congestion, or cardiomegaly appear, the possibility of pericarditis with effusion should be considered. Differentiating effusive pericarditis from cardiac enlargement due to other causes is often challenging. Signs favoring the diagnosis of the former include jugular vein distension with paradoxical pulse, weak apical impulse, faint heart sounds, absence of valvular murmurs, and an early diastolic extra sound; X-ray or kymography showing loss of normal cardiac contour and weak pulsations; and ECG showing low voltage, ST-T changes without QT prolongation. Further diagnostic tests may include ultrasound, radionuclide scanning, and MRI, while pericardiocentesis and pericardial biopsy can aid in definitive diagnosis. The severe pain of nonspecific pericarditis closely resembles acute myocardial infarction, but the former often has a history of upper respiratory infection before onset, pain significantly aggravated by breathing, coughing, or positional changes, early appearance of pericardial friction rub, and normal serum aspartate aminotransferase, lactate dehydrogenase, and creatine phosphokinase levels, with no abnormal Q waves on ECG; the latter typically occurs in older patients, often with a history of colicky pain or myocardial infarction, pericardial friction rub appearing 3-4 days after onset, ECG showing abnormal Q waves, ST segment elevation with upward convexity, and T wave inversion, often accompanied by severe arrhythmias and conduction blocks. If the pain of acute pericarditis is mainly in the abdomen, it may be misdiagnosed as an acute abdomen, but detailed history taking and physical examination can prevent misdiagnosis. The clinical manifestations and treatment of pericarditis vary depending on the underlying disease cause.
bubble_chart Treatment Measures
The treatment of acute pericarditis includes addressing the primary disease cause, relieving cardiac tamponade, and symptomatic treatment. Patients should rest in bed. Sedatives should be administered for chest pain, and morphine or left stellate ganglion block may be used if necessary. For wind-dampness pericarditis, anti-wind-dampness treatment should be intensified, with a preference for corticosteroids (see the chapter on "Acute Wind-Dampness Heat"). For subcutaneous node pericarditis, anti-subcutaneous node treatment should be initiated as early as possible, with sufficient dose and a prolonged course until about one year after the cessation of subcutaneous node activity (refer to the chapter on "Subcutaneous Node Disease"). If symptoms of cardiac tamponade appear, pericardiocentesis should be performed; if effusion continues or constrictive pericarditis is suspected, timely pericardiectomy should be considered to prevent progression to constrictive pericarditis. For purulent pericarditis, adequate antibiotics effective against the causative bacteria should be selected, and repeated pericardiocentesis for pus drainage and intrapericardial antibiotic injection should be performed. If the response is poor, early consideration should be given to pericardial incision and drainage. If pericardial thickening is found during drainage, extensive pericardiectomy may be performed. For nonspecific pericarditis, corticosteroids may be effective, and pericardiectomy may be considered for recurrent cases. When pericardial effusion causes cardiac tamponade, pericardiocentesis should be performed, preferably after ultrasound localization of the puncture site and direction. The puncture needle should be connected to a reliable ECG machine's chest lead electrode for monitoring. Atropine should be used prophylactically to avoid vagal hypotension. There are two common puncture sites (Figure 1): ① The angle between the xiphoid process and the left costal margin, with the needle tip directed slightly upward and backward, closely following the posterior surface of the sternum. The patient should be in a semi-recumbent position. This site is particularly suitable for small effusions, as it is less likely to injure coronary vessels, provides good drainage, and avoids the pleural cavity, making it especially suitable for purulent pericarditis to avoid contamination. ② 1-2 cm inside the left fifth intercostal space within the cardiac dullness border, with the needle tip directed backward and inward toward the spine. The patient should be in a sitting position. Aseptic technique should be observed, and the needle should be advanced slowly. If cardiac pulsation is felt, the needle should be slightly withdrawn. Fluid should not be aspirated too quickly, and an appropriate amount of antibiotics can be injected into the pericardial cavity after aspiration.
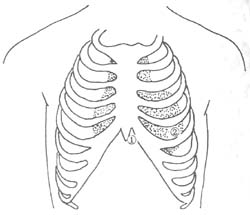
Figure 1 Common sites for pericardiocentesis.
The prognosis is primarily determined by the underlying disease cause. For instance, if it is complicated by acute myocardial infarction, malignant tumors, or systemic lupus erythematosus, the prognosis is severe. In cases such as subcutaneous nodular or purulent pericarditis, timely and effective treatment, including necessary pericardiocentesis or pericardiotomy to drain pus, can lead to a full recovery. However, some cases may result in residual myocardial damage and progress to constrictive pericarditis.
After the diagnosis of acute pericarditis is established, it is necessary to further clarify its disease cause to provide direction for treatment. The differential diagnosis of four common types of acute pericarditis is shown in Table 1.
Table 1 Differential Diagnosis of Four Common Types of Pericarditis
| Wind-dampness Pericarditis | Subcutaneous Node Pericarditis | Purulent Pericarditis | Non-specific Pericarditis | ||
| Medical History | Often preceded by upper respiratory tract infection 1-2 weeks before onset, accompanied by other Bi disease manifestations, as part of pancarditis | Often accompanied by primary subcutaneous node lesions, or coexisting with other serous membrane cavity subcutaneous nodes | Often has primary infectious lesions, accompanied by obvious toxemia manifestations | Often preceded by upper respiratory tract infection 1-2 weeks before onset, onset is usually acute, may recur | |
| Fever | Mostly irregular grade I or grade II fever | Low-grade fever or often not significant | High fever | Persistent fever, either continuous or remittent | |
| Chest Pain | Often present | Often absent | Often present | Often extremely severe | |
| Pericardial Friction Rub | Often present | Rarely present | Often present | Prominent, appears early | |
| Heart Murmur | Often accompanied by significant murmurs | Absent | Absent | Absent | |
| Anti-streptolysin O Titer | Often elevated | Normal | Normal or elevated | Normal or elevated | |
| White Blood Cell Count | Grade II elevation | Normal or grade I elevation | Significantly elevated | Normal or elevated | |
| Blood Culture | Negative | Negative | May be positive | Negative | |
| Pericardial Effusion | Amount | Usually small | Often large | Moderate | Small to moderate |
| Nature | Mostly straw-colored | Mostly bloody | Purulent | Straw-colored or bloody | |
| ADA activity | <30U/L | ≧30U/L | <30U/L | <30U/L | |
| Cell classification | Neutrophils predominate | Lymphocytes are more numerous | Neutrophils predominate | Lymphocytes predominate | |
| Bacteria | None | Sometimes subcutaneous node bacilli are found | Pyogenic bacteria can be found | None | |
| Pericardial air insufflation | Cardiac enlargement | No cardiac enlargement | No cardiac enlargement | Cardiac enlargement is common | |
| Treatment | Anti Bi disease drugs | Anti subcutaneous node drugs | Antibiotics | Adrenocortical hormones | |




