| disease | Hemolytic Disease of the Newborn |
| alias | Congenital Neonatal Severe Jaundice, Fetal Erythroblastosis, Fetal Generalized Edema |
Hemolytic disease of the newborn (HDN) has completely changed the perception of being rare with the advancement of neonatal immunology in our country. Large maternity and child health hospitals and pediatric newborn disease wards detect this condition almost every month or even every week. Before the disease was understood, it was referred to as "fetal generalized edema," "fetal erythroblastosis," "congenital severe neonatal jaundice," or "neonatal hemolytic disease." Now, with a clear understanding of its pathological mechanism rather than just symptoms, it has been uniformly named "hemolytic disease of the newborn."
bubble_chart Etiology
This disease is primarily a hereditary condition caused by blood group incompatibility between mother and infant, leading to an isoantigen immune reaction. The dominant antigen inherited by the fetus from the parents happens to be absent in the mother. This antigen invades the maternal body, producing immune antibodies that pass through the placental chorionic membrane into the fetal bloodstream, causing agglutination and destruction of fetal red blood cells, resulting in hemolysis. This leads to anemia, edema, hepatosplenomegaly, and the rapid onset of progressive grade III jaundice shortly after birth, and may even cause bilirubin encephalopathy.
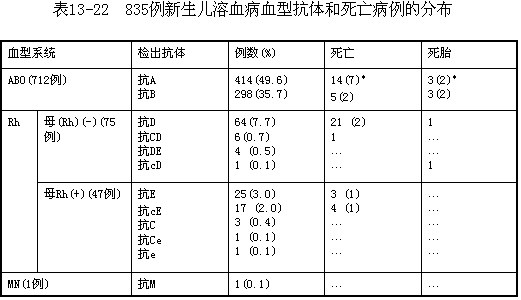
To date, 26 blood group systems and over 400 blood types have been identified in humans. Each blood group system is genetically independent and follows Mendelian inheritance. Neonatal hemolytic disease is most commonly caused by ABO incompatibility, followed by the Rh system. Other systems, such as MN, Kell, and Duffy, are less frequently implicated. Among the 835 cases observed in Shanghai over the past 18 years, ABO incompatibility accounted for 85.3%, Rh incompatibility for 15.6%, and MN incompatibility for 0.1%. The distribution of various antibodies and their association with dead fetus and neonatal death is shown in Table 1.
**Appendix 1: ABO Incompatibility** Most cases occur when the father is type A, B, or AB, and the mother is type O, with the former carrying dominant antigens and the latter lacking them. A minority of cases arise when the mother is heterozygous for type A or B, and the father's dominant antigen enters the heterozygous mother (lacking that antigen) and combines with an O gene-carrying ovum, leading to the disease. Whether subsequent pregnancies are affected depends on whether the father's gene is homozygous or heterozygous. Since the mother's blood may contain natural antibodies (α or β agglutinins), the disease can manifest in the first pregnancy. Due to the relatively weak antigenicity of A (B) antigens, the anti-A (B) antibodies entering the fetal body are partially neutralized by blood group substances and adsorbed by tissue cells, so only a minority of ABO-incompatible mother-infant pairs develop the disease.
**Appendix 2: Rh Incompatibility** The Rh blood group system comprises six antigens, divided into three pairs: Cc, Dd, and Ee. Any one antigen from each pair, totaling three antigens, forms a gene complex. Each individual has two gene complexes, one inherited from each parent. Individuals lacking the D antigen in both gene complexes are Rh-negative, while those with the D antigen are Rh-positive. Heterozygotes carry one D antigen, and homozygotes carry two. A study of 50,000 Han Chinese in Shanghai revealed that 0.34% were Rh-negative, while approximately 4.96% of Uyghurs were Rh-negative.
The d antigen is purely theoretical, as no anti-d antibody has been discovered to date. Among Rh antigens, D is the most potent. As shown in the table, most mothers of Rh hemolytic disease cases are Rh-negative, and this group also accounts for the highest incidence of dead fetus and neonatal death. However, children of Rh-positive mothers can also develop the disease, albeit with different blood group categories, with anti-E being the most common.
When maternal and fetal blood types are incompatible, the mother may be immunized, but it is not inevitable. After reviewing the literature, Woodrow stated that approximately 8% of mothers show a significant risk of Rh immunization within 6 months after the first childbirth of an Rh-positive, ABO-compatible baby. During the second Rh-positive pregnancy (having been sensitized by the first), the occurrence of Rh antibodies is also 8%. Therefore, the total risk of Rh immunization after the first ABO-compatible, Rh-positive pregnancy is approximately 16%.
In cases of ABO incompatibility and Rh-positive pregnancy, the risk of Rh immunization is very small, only 10-20% of that in ABO-compatible, Rh-positive pregnancies, meaning the unexplained cause is only about 2%. This may be because when ABO incompatibility occurs, the red blood cells entering the maternal bloodstream undergo hemolysis, and their Rh antigens are destroyed in the liver.
It is primarily caused by the destruction and hemolysis of fetal red blood cells due to antigen-antibody reactions. ① Increased red blood cell destruction leads to compensatory hyperplasia of bone marrow and extramedullary hematopoiesis, with extramedullary hematopoietic foci scattered in organs such as the liver and spleen. ② The reticuloendothelial system, as well as liver and kidney cells, may exhibit hemosiderin deposition. ③ Anemia results in cardiac enlargement, and hypoxia increases vascular wall permeability, accelerating fluid leakage, leading to edema and serous membrane cavity effusion. ④ In grade III hemolysis, the fetus excretes large amounts of bilirubin, which can cause amniotic fluid, amniotic membrane, umbilical cord, and vernix caseosa to exhibit conjugated hyperbilirubinemia. This can lead to systemic jaundice and bilirubin encephalopathy, commonly affecting areas such as the basal ganglia, subthalamic nucleus, and caudate nucleus.
bubble_chart Clinical Manifestations
The symptoms of hemolytic disease caused by ABO and Rh incompatibility are fundamentally similar, differing mainly in severity—the former being milder and progressing more slowly, while the latter is more severe and advances rapidly.
In mild cases, newborns appear normal at birth but gradually develop jaundice and anemia within 1–2 days, with symptoms worsening over time. Some may exhibit slight drowsiness or refusal to feed, making such cases easily misdiagnosed as physiological jaundice.
In severe cases, extensive destruction of fetal red blood cells leads to anemia, generalized edema, pleural and peritoneal effusions, and hepatosplenomegaly, resulting in stillbirth, late miscarriage, or premature labor. Some infants die shortly after birth due to anemia, edema, or heart failure. Jaundice is rare in utero because bilirubin from red blood cell breakdown is excreted via the placenta. After birth, the timing and progression of jaundice depend on the intensity of antibody-mediated red blood cell destruction. Earlier onset and faster progression indicate more severe disease, with jaundice deepening rapidly. Corresponding symptoms include drowsiness, refusal to feed, weakening of the Moro reflex, worsening anemia and hepatosplenomegaly, and a shift in jaundice hue from bright yellow to golden yellow. Without prompt treatment, serum-free unconjugated bilirubin levels exceeding 342 μmol/L (20 mg/dL) can cause kernicterus, characterized by loss of the Moro reflex, high-pitched crying, tonic spasms, convulsions, and opisthotonos, often leading to death. Such cases were historically misdiagnosed as neonatal sepsis. Survivors may suffer long-term sequelae like intellectual disability, motor impairments, and hearing loss.
If the disease is suspected prenatally, attention should be paid at birth to placental edema, with a placenta-to-neonate weight ratio as high as 1:7 or even 1:1.3–4, and yellow-tinged amniotic fluid.
bubble_chart Auxiliary Examination
Decreased red blood cells, reduced hemoglobin, significantly increased reticulocytes, and nucleated red blood cells observed in the smear. The white blood cell count may be substantially elevated due to the inclusion of nucleated red blood cells. These blood picture changes also vary with the severity of hemolysis.
When there is Rh blood group incompatibility between mother and infant, using horse serum to determine the ABO blood type may lead to misidentification. This is because, under the stimulation of human red blood cell surface antigens, horses produce anti-A (B) antibodies as well as anti-IgG antibodies. Therefore, if unexplained discrepancies arise, the possibility of this condition should be considered, and human serum should be used instead for ABO blood typing.
Specific antibody tests reveal immune antibodies. The serum jaundice index increases, and bilirubin levels rise, with results potentially differing by more than threefold due to variations in methodology. Urinary and fecal urobilinogen excretion increases. When the bile ducts are obstructed by bile plugs, stools may appear grayish-white, and bilirubin may be detected in the urine. In ABO hemolytic disease, the activity of red blood cell acetylcholinesterase is significantly reduced. Plasma albumin, prothrombin, and fibrinogen levels may decrease, all of which can contribute to hemorrhagic symptoms. In severe cases, thrombocytopenia, prolonged bleeding time, and poor clot retraction may occur. A few cases may develop DIC.
1. Medical History Pregnant women with a history of unexplained dead fetus, late abortion, blood transfusion, or severe neonatal jaundice, or those whose newborns show progressive deepening of jaundice shortly after birth, should undergo specific antibody testing. Specimen submission requirements are as follows: ① Tubes must be clean and dry to prevent hemolysis. ② For prenatal blood group antibody testing, submit blood samples from the mother and her husband; for neonatal testing, prioritize the newborn’s blood sample, supplemented by parental samples (if the mother’s blood cannot be obtained promptly, only the newborn’s sample may be submitted). ③ Draw 3ml of blood from the newborn (non-anticoagulated); 5ml from the mother (non-anticoagulated); and 2ml from the husband (anticoagulated, using standard anticoagulants). ④ If local testing is unavailable, separate the mother’s serum and mail it to a nearby testing facility, along with an additional 2ml of anticoagulated blood. In hot weather, place the blood sample vial in a wide-mouthed bottle with ice and send it by airmail (ensure sterile conditions during transit).
2. Blood Type During pregnancy, fetal BO blood type can be determined via amniotic fluid. If mother and fetus are confirmed to share the same type, there is no risk of immunization. However, Rh blood type lacks blood group substances and requires fetal blood for typing. A newborn with type O blood can rule out ABO hemolytic disease but not hemolytic disease caused by other blood group systems.
3. Specific Antibody Testing This includes blood typing of the mother, infant, and father; antibody titers; the antiglobulin test (indirect method prenatally, direct method postnatally); and the release and free antibody tests, which are the primary diagnostic criteria for this condition.
The indirect antiglobulin test uses red blood cells with known antigens to detect incomplete antibodies in the patient’s serum. A positive direct test indicates that the infant’s red blood cells have been sensitized by blood group antibodies. A positive release test confirms the diagnosis, as sensitized red blood cells release antibodies upon heating, and the specificity of the antibodies in the released solution can be determined using standard red blood cells. The free antibody test detects incompatible antibodies in the newborn’s serum that have not yet sensitized red blood cells; a positive result suggests potential harm.
For cases suspected prenatally, antibody titers should be checked monthly before the 6th month of pregnancy, biweekly during the 7th–8th months, and weekly after the 8th month or as needed. Fluctuations in antibody titers (from low to high or a sudden drop from high to low) indicate unstable conditions and possible worsening. Stable titers suggest a stable condition or maternal-fetal blood type compatibility, with the antibodies being remnants from prior exposure. Excluding residual factors, disease severity generally correlates with antibody titer levels. However, in the ABO system, due to naturally occurring anti-A(B) substances, some unmarried women may already have titers as high as 1024. Typically, an ABO hemolytic disease titer of 64 is considered suspicious, though cases have been reported even at a titer of 8.
4. Amniotic Fluid Bilirubin Testing Unlike antibody titers, amniotic fluid bilirubin levels are unaffected by residual factors from previous pregnancies. Thus, they provide valuable guidance for assessing disease severity and determining the timing for pregnancy termination. Normal amniotic fluid is clear and colorless, while in severe hemolytic disease, it appears yellow.
Liley found that the increase in optical density at 450nm correlates with the severity of fetal hemolytic disease. Since normal fetal amniotic fluid bilirubin concentration decreases with gestational age, the significance of the optical density increase at 450nm varies by gestational week. Liley plotted an empirical graph based on results from 101 Rh-immunized women, with the optical density increase at 450nm on the vertical axis and gestational age on the horizontal axis, dividing it into three zones. Cases in the upper zone indicate severe disease, often fatal. Those in the middle zone represent grade II severity, while those in the lower zone may involve Rh-negative infants or Rh-positive infants with very mild anemia, with only 10% requiring exchange transfusion.
5. Imaging Examination: Generalized edema in the fetus is visible on X-ray films as widened translucent bands in the soft tissues, with reduced curvature of the limbs. Ultrasound examination provides clearer images, showing hepatosplenomegaly and effusions in the thoracic and abdominal cavities, all of which can be displayed on the screen.
6. Other laboratory tests are also of reference value for the diagnosis of this disease.
bubble_chart Treatment Measures
1. Fetal Treatment For sensitized pregnant women, Peking Union Medical College Hospital uses a mixture of motherwort herb 500g, Chinese Angelica 250g, Sichuan Lovage Rhizome 250g, Peony Root 300g, and Aucklandia Root 12g, ground into fine powder and made into honey pills, each weighing 9g. During pregnancy, take 1–3 pills daily until childbirth. The China International Peace Maternity and Child Health Hospital and Peking Union Medical College Hospital administer jaundice Virgate Wormwood infusion granules (containing Virgate Wormwood, prepared Rhubarb Rhizome, Skullcap Root, Liquorice Root, etc.) orally to pregnant women with Rh or ABO incompatibility. This has shown some efficacy in preventing late abortion, fetal death, premature labor, and alleviating neonatal symptoms.
Comprehensive Western medical treatment (vitamin K 2mg once daily, vitamin C 500mg plus 25% glucose 40ml intravenously once daily, oxygen inhalation twice daily for 20 minutes each, and vitamin E 30mg three times daily throughout pregnancy) administered for 10 days during early, middle, and late pregnancy can reduce the incidence of dead fetus, late abortion, premature labor, and alleviate neonatal symptoms.
As pregnancy progresses closer to term, more antibodies are produced, increasing the impact on the fetus and the risk of death. If there is a history of dead fetus or if Rh antibody titers rise from low to 1:32–64 or suddenly drop from high levels; if fetal heart sounds show murmurs, excessive abdominal circumference or weight gain in late pregnancy, general weakness, poor appetite, elevated amniotic fluid bilirubin, or imaging findings such as edema, ascites, or hepatosplenomegaly, early termination of pregnancy should be considered. Induction is typically performed at 35–38 weeks, aiming for an L/S ratio ≥2. Oral phenobarbital for one week (10–30mg three times daily) can reduce RDS, enhance fetal liver enzyme activity, and alleviate postnatal jaundice. ABO incompatibility is generally milder and rarely requires early termination of pregnancy.
For severe cases where amniotic fluid optical density suggests possible fetal death, intrauterine blood transfusion may be considered starting at 20 weeks, repeated every 2 weeks initially and then every 3–4 weeks. Blood is injected into the fetal peritoneal cavity to correct anemia and improve survival chances. The transfusion volume is calculated as (gestational age – 20) × 10. Excessive transfusion volume or abdominal pressure exceeding umbilical vein pressure can cause circulatory arrest and fetal death. However, this method carries risks of infection, bleeding, premature labor, and may stimulate the placenta to allow more fetal blood to flow into the mother, worsening the condition, so it is generally not used.
In collaboration with the Shanghai Blood Center, the Shanghai First Maternity and Infant Health Hospital has applied domestically produced blood component separators since 1981 for plasma exchange therapy in severe Rh cases with recurrent dead fetus or neonatal systemic edema, achieving satisfactory results. The goal is to remove antibodies, reduce titers, minimize hemolysis, and improve fetal survival. Typically, 100ml of plasma is exchanged weekly starting at 20 weeks of gestation or as needed based on the condition. Skin itching or protein allergies may occur during the process but can be resolved with symptomatic treatment.
2. Management During Labor Prepare blood donors, equipment, and transfusion personnel as much as possible. For ABO incompatibility, natural delivery at full term is preferable, while Rh incompatibility requiring early termination of pregnancy may necessitate a cesarean section. Since red blood cells are already damaged in utero, hypoxia is more pronounced, increasing the risk of asphyxia at birth, so precautions are essential. The umbilical cord should be clamped immediately after delivery to prevent excessive umbilical bleeding into the infant, which could worsen the condition. Leave a 5–6cm stump when cutting the cord, ligate the distal end, wrap it in sterile gauze, and soak it in 1:5000 furacilin solution to keep it moist for potential exchange transfusion. After wiping maternal blood from the placental end of the cord, allow 3–5ml of cord blood to flow into a sterile tube for specific antibody and serum bilirubin testing, along with routine blood tests, blood typing, and nucleated red blood cell counts. Squeezing the cord may introduce gelatinous material into the blood, affecting the accuracy of the Coombs test. The placenta should be weighed and sent for pathological examination—the heavier the placenta, the more severe the condition.
3. Treatment of Newborns The focus at birth is on preventing and treating anemia and heart failure. For those with anemia, generalized edema, ascites, or heart failure, concentrated blood transfusion should be performed immediately after draining ascites and withdrawing 30–50 ml of blood from the umbilical vein. The focus during days 2–7 after birth is on preventing and treating jaundice and bilirubin encephalopathy. Severe anemia should be monitored within 2 months.
For the management of jaundice and hyperbilirubinemia, phototherapy and Chinese-Western medications can alleviate most cases. However, exchange transfusion is still necessary to promptly remove antibodies, reduce ongoing red blood cell destruction, lower bilirubin levels, correct anemia, improve hypoxia, and prevent heart failure. Its efficacy surpasses that of phototherapy and medications, but it requires significant manpower and resources and carries risks such as thrombosis, air embolism, cardiac arrest, and potential infection. Therefore, strict indications must be followed.
(1) Indications for exchange transfusion: ① Neonates with umbilical cord hemoglobin below 120g/L (12g%) at birth, accompanied by edema, hepatosplenomegaly, or congestive heart failure. ② Serum bilirubin reaching 342μmol/L (20mg/dl), or in larger, well-conditioned infants without drowsiness or feeding refusal, up to 427.5μmol/L (25mg/dl) or higher. ③ Any signs of bilirubin encephalopathy. ④ Premature labor or severe disease in a previous sibling may warrant looser indications.
(2) Blood type selection: For Rh hemolytic disease, use ABO-compatible (or type O) Rh-negative heparinized blood. If special frozen blood is available, it can be used after thawing. In emergencies, Rh-positive blood without anti-D antibodies may be used (preferably from untransfused male donors or non-pregnant female donors). For ABO hemolytic disease, use a mixture of AB plasma and type O red blood cells.
(3) Anticoagulants: Add 3–4mg heparin per 100ml blood for effective anticoagulation. After transfusion, protamine (half the heparin dose) can neutralize it. Citrate-based anticoagulants dilute blood by 1/5, impairing anemia correction and binding free calcium, which may cause hypocalcemia. Therefore, slowly inject 1ml of 10% calcium gluconate per 100ml blood exchanged, followed by 2–3ml at the end.
(4) Exchange transfusion procedure: Pre-transfusion phototherapy and IV albumin/plasma can enhance bilirubin removal. Skip one feeding or aspirate gastric contents to prevent vomiting. Sedation with phenobarbital sodium IM or chloral hydrate PO may be used if necessary. Perform the procedure in an operating room at ~25°C. Pre-warm the donor blood to 37°C using a spiral warmer. Position the neonate supine, expose the abdomen, and secure limbs with padded splints. After skin disinfection, cover with sterile drapes. Local anesthesia is needed for venous cutdown. Pre-assemble siliconized syringes, multi-way stopcocks, and tubing, then lubricate with heparinized saline (200ml saline + 0.1ml heparin). Connect inflow/outflow tubing and prepare a waste blood basin. Discontinue IV fluids during the procedure to avoid interference.
(5) Umbilical vein exchange transfusion: If the umbilical cord is intact, trim to ~5cm. The thin-walled, large-lumen umbilical vein is visible; insert the catheter at a 30° angle upward and rightward. If difficult, use a probe to guide insertion. For detached cords, remove the scab and attempt insertion. If unusable, make a 1.5cm semicircular incision 1cm above the umbilicus under local anesthesia, dissect soft tissue, incise the fascial membrane, and locate the ~0.5cm-wide grayish-white umbilical vein slightly right of midline. Incise the outer collagenous membrane, free the vein, cannulate 4–6cm while aspirating, and secure the catheter once blood flows freely. Collect initial and final samples for bilirubin testing.
After exchanging an equal volume of anticoagulated blood, lift the catheter vertically to measure venous pressure, reducing clotting risk. Measure every 100ml exchanged; if venous pressure exceeds 8cmH 2 O, withdraw more than infuse to avoid ischemic shock. Generally, the input-output difference should not exceed 30–50ml.
The exchange blood volume is calculated at 150-180 ml/kg, approximately twice the total blood volume of the infant, with a total volume of about 400-600 ml. This amount can replace about 85% of the sensitized red blood cells. Each withdrawal and infusion should be 20 ml, with a uniform speed of about 10 ml per minute. Drawing too quickly may cause the side hole of the catheter to adhere to the vein wall, preventing successful withdrawal. Additionally, it takes time for bilirubin in the tissues to re-enter the blood vessels, so there is no need to rush. For infants with low body weight, severe conditions, significant anemia, or heart failure, the withdrawal and infusion volume should be halved each time to reduce fluctuations in venous pressure. The total exchange volume may also be appropriately reduced, and concentrated blood with half the plasma can be used. During the actual exchange, two long needles (plasma collection needles) are used in the blood bottle. The air inlet needle should penetrate the blood surface, while the blood withdrawal needle can be adjusted as needed. The upper layer of plasma is used first, followed by the lower layer of blood cells, or the lower layer of blood cells can be drawn directly. Generally, by the end of the exchange, more blood cells are infused to reduce postoperative anemia. During the exchange, the catheter must be replaced promptly if necessary and rinsed in heparinized saline. If the issue is related to the catheter, slightly adjust its insertion depth. If blockage is suspected, replace the catheter and reinsert it vertically.
The blood exchange is completed. Remove the catheter and check each channel for clotting. The distal end of the umbilical cord is double-ligated and wrapped with sterile gauze, then moistened with 1:5000 nitrofurazone solution to keep it wet for potential reuse. If an incision is made above the umbilicus, the umbilical vein is ligated, and the fascia and skin are sutured, followed by sterile dressing.
⑹ Synchronous exchange: Insert two catheters—one into the umbilical artery for withdrawal and the other into the umbilical vein for infusion, performed simultaneously. The advantages include reduced venous pressure fluctuations, avoidance of wasting approximately 1ml of fresh blood in the catheter during each withdrawal and infusion, and shortened exchange time. The drawback is the insertion of an additional catheter, increasing the risk of perforation, bleeding, and infection. During the procedure, the umbilical artery catheter must be inserted first, directed downward at a 45° angle to the abdominal wall, and carefully maneuvered through three physiological bends: the umbilical ring (about 2cm), the attachment point of the bladder wall (about 4cm), and the entrance of the internal iliac artery (about 7cm). If resistance is encountered, gently rotate and advance or withdraw slightly before reinserting. Avoid forceful insertion to prevent perforation. If unsuccessful, attempt insertion into the other umbilical artery, ensuring the catheter tip reaches approximately 14cm to the level of the fourth lumbar vertebra (confirmed by X-ray). The umbilical vein is wider and easier to cannulate, similar to umbilical vein exchange, inserted about 6cm until blood flows freely. Inserting the umbilical vein first may cause spasm of the umbilical artery, making insertion difficult. To prepare for potential reuse, heparinized solution can maintain catheter patency, but strict infection control is essential. When removing the umbilical artery catheter, pause briefly at 2cm from the exit to stimulate contraction of the proximal segment before complete withdrawal to minimize bleeding.
⑺ Post-exchange care: Continue phototherapy and intensive monitoring. Measure heart rate and respiration every 4 hours, and monitor jaundice severity along with symptoms like drowsiness, refusal to feed, dysphoria, spasms, and the Moro reflex. Discontinue monitoring once jaundice subsides. Administer vitamins for 3 days to prevent infection. After suture removal, transition to routine care and resume breastfeeding.
Perform blood tests, including nucleated red blood cell counts, every 1–3 days, and measure bilirubin daily until jaundice resolves. After discharge, monitor red blood cells and hemoglobin every two weeks for two months. If hemoglobin falls below 70g/L (7g/dl), administer small transfusions to correct anemia. Early and adequate oral iron supplementation during recovery may shorten the duration and severity of anemia.
After a single exchange, bilirubin from extravascular tissues may re-enter the plasma. Combined with hemolysis of sensitized red blood cells and the breakdown of transfused cells, serum bilirubin may rise again. If indicated, consider another exchange. In the past, up to four exchanges were required to save a patient, but with phototherapy, the need for exchanges—or even a second one—has decreased.
Mild cases can recover quickly with just glucose supplementation and no special treatment. Severe cases, if treated promptly after birth, can also improve rapidly and show no difference from normal children as they grow. Early-stage bilirubin encephalopathy may still be cured after blood exchange, while advanced-stage cases often leave sequelae. For those with systemic edema, even with aggressive treatment, the chances of success are low.
In recent years, utilizing the theory of passive immunity, anti-D IgG immunoglobulin has been produced. For Rh-negative, non-immunized women who deliver their first Rh-positive newborn, an intramuscular injection of 300μg is administered within 72 hours to neutralize the D antigen entering the maternal body. It is also required after amniocentesis or late abortion. Its effectiveness in suppressing Rh immune responses is excellent, with a failure rate of approximately 1.5% to 2.0%. In China, due to the relatively low incidence of Rh blood type incompatibility, few women know they are Rh-negative before their first pregnancy. Therefore, although the Shanghai Central Blood Station has prepared it, its actual usage is very limited.
Avoiding unnecessary blood transfusions can reduce the incidence of this condition.
It should be differentiated from various other diseases that can cause the same symptoms.
1. Generalized edema should be distinguished from hereditary globin peptide chain synthesis disorders such as α-thalassemia (Hb Bant's fetal edema type) and congenital malformations. Other factors to consider include maternal diabetes, congenital nephropathy, placental insufficiency, twin-to-twin or fetomaternal transfusion, and intrauterine infections. These can all be differentiated through clinical and serological examinations.
2. Jaundice: Physiological jaundice appears late, progresses slowly, is mild in severity, and is not accompanied by anemia or hepatosplenomegaly. Sepsis presents with toxic symptoms, fever, and negative specific antibodies, with blood culture aiding in differentiation. Other conditions to consider include cytomegalic inclusion disease, toxoplasmosis, intracranial hemorrhage, G-6-PD deficiency, and other congenital hemolytic diseases.
3. Anemia: Primarily differentiated from blood loss anemia caused by various factors. G-6-PD deficiency is more common in southern regions. Other congenital hemolytic anemias and nutritional anemias are relatively rare.






