| disease | Ischemic Heart Disease |
Ischemic heart disease includes coronary artery obstruction or stenosis caused by atherosclerotic lesions. Left ventricular aneurysm due to myocardial ischemia, ventricular septal defect after myocardial infarction, and mitral valve insufficiency caused by papillary muscle ischemia are common acquired heart diseases in middle-aged and elderly individuals. This section focuses on coronary artery atherosclerotic obstruction or stenosis. Atherosclerotic lesions in the coronary artery walls lead to narrowing of the vessel lumen, obstructing coronary circulation and causing insufficient myocardial blood supply. Severe obstruction can lead to myocardial infarction. Over the past 40 years, the incidence of coronary atherosclerotic heart disease has gradually increased in China. According to statistics from Shanghai Medical University, coronary heart disease accounted for only 6% of hospitalized heart disease cases from 1948 to 1958; it rose to 18% from 1959 to 1971; and increased to 29% from 1972 to 1979. Currently, it ranks first among various types of heart diseases.
bubble_chart Etiology
The pathogenesis of coronary atherosclerosis is relatively complex and has not yet been fully understood. Based on extensive epidemiological and experimental research data, the main disease-causing factors include: high-calorie, high-fat, high-sugar diets, smoking, hyperlipidemia, hypertension, diabetes, obesity, insufficient physical activity, intense mental labor, emotional excitability, mental stress, middle-aged and elderly males, low high-density lipoprotein, and abnormal coagulation function. A small number of cases may have familial genetic factors.
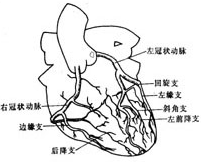
bubble_chart Pathological ChangesCoronary artery anatomy: The coronary artery is a blood vessel that supplies blood and oxygen to the myocardium, and its anatomical morphology is quite variable. Under normal circumstances, the coronary artery has left and right branches, which open into the left and right coronary sinuses of the ascending aorta, respectively. Sometimes, a smaller accessory coronary artery arises from the aorta.
The main trunk of the left coronary artery is approximately 4-5 mm in diameter and about 0.5-2 cm in length. After arising from the ascending aorta, it runs leftward and downward behind the pulmonary trunk, and between the pulmonary trunk and the left auricle, it divides into the anterior descending branch and the circumflex branch along the left atrioventricular groove.
The anterior descending branch is the continuation of the main trunk of the left coronary artery. It descends along the anterior interventricular groove, then curves around the cardiac apex notch to reach the posterior wall of the heart, where it anastomoses with the posterior descending branch of the right coronary artery at the lower third of the posterior interventricular groove. The anterior descending branch gives off the left conus branch, diagonal branch, left anterior ventricular branch, right anterior ventricular branch, and anterior interventricular septal branch, supplying blood to the root of the aorta and pulmonary trunk, part of the left atrial wall, the anterior wall of the left ventricle, part of the anterior wall of the right ventricle, most of the interventricular septum (upper and anterior parts), the apex region, and the anterior papillary muscle.
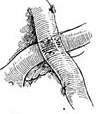
The circumflex branch arises from the main trunk of the left coronary artery and runs leftward and backward along the anterior part of the left atrioventricular groove, close to the base of the left auricle, descending along the left border of the heart to reach the diaphragmatic surface. The circumflex branch has many variations in its branches, mainly including several left marginal branches, left posterior ventricular branches, and atrioventricular branches along the left atrioventricular groove. The atrioventricular branch is sometimes (about 10%) longer and gives off the posterior descending branch and the atrioventricular nodal artery from its end. In 30% of individuals, the circumflex branch also gives off the sinoatrial nodal artery. The circumflex branch supplies blood to the lateral and posterior walls of the left ventricle, the left atrium, and sometimes also to the diaphragmatic surface of the ventricle, the anterior and posterior papillary muscles, part of the interventricular septum, the atrioventricular node, the atrioventricular bundle, and the sinoatrial node.The right coronary artery arises from the right coronary sinus and runs close to the base of the right auricle, descending outward and downward along the right atrioventricular groove. Upon reaching the junction of the ventricle, atrium, and interatrial and interventricular septa at the posterior part of the atrioventricular groove, it divides into two branches: the right posterior descending branch runs toward the apex region along the posterior interventricular groove, and the smaller atrioventricular nodal artery turns upward. The main branches of the right coronary artery include the right conus branch, right atrial branch, sinoatrial nodal branch, right anterior ventricular branch, right posterior ventricular branch, posterior interventricular septal branch, posterior descending branch, and atrioventricular nodal artery. The right coronary artery supplies blood to the right atrium, sinoatrial node, right ventricular outflow tract, pulmonary conus, anterior and posterior walls of the right ventricle, the lower third of the interventricular septum, and the atrioventricular node. In patients with right coronary artery dominance, it also supplies blood to part of the left ventricle and the apex. The distribution areas of the left and right coronary arteries on the diaphragmatic surface of the myocardium are quite variable. When a coronary artery branch with a larger blood supply area develops stenosis, the area of myocardial ischemia injury is wider, and the condition is more severe (Figure 3).
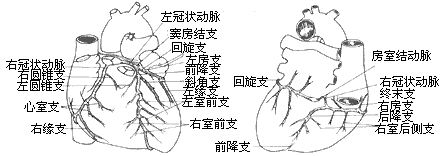
(1) Anterior view (2) Posterior view
(3) Right anterior oblique view (4) Left anterior oblique view
Figure 3 Coronary artery anatomy
[Classification]
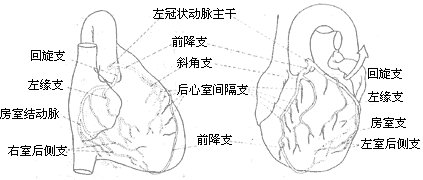
Based on the blood supply sources at the crux of the heart, i.e., the junction of the ventricles, atria, and atrioventricular septum on the posterior wall of the heart, the distribution of the left and right coronary arteries can be divided into three main types:
1) Right coronary artery dominance: This type is the most common, accounting for about 80%. The right coronary artery is thick and long, supplying blood to the posterior wall of the right ventricle and, via the posterior descending branch, to part of the posterior wall of the left ventricle and the posterior part of the interventricular septum.
2) Left coronary artery dominance: The right coronary artery is smaller, and the circumflex branch of the left coronary artery gives off the posterior descending branch, supplying blood to the posterior walls of the left and right ventricles and the interventricular septum.
3) Left and right coronary stirred pulse balanced type. The left and right coronary stirred pulses each emit a posterior descending branch to supply blood to the posterior walls of the left and right ventricles (Figure 4).
(1) Right coronary stirred pulse dominant type
(2) Left coronary stirred pulse dominant type
(3) Balanced type of left and right coronary stirred pulse
Figure 4 Distribution types of left and right coronary stirred pulse
Pathological anatomy: Atherosclerotic lesions of the coronary stirred pulse mostly occur in the proximal segments of the main branches of the coronary stirred pulse, within about 5cm from the opening of the main stirred pulse, often located in the atrioventricular groove, surrounded by fat tissue of the main branches of the coronary stirred pulse, providing favorable conditions for surgical treatment. In patients with hypertension or diabetes, the lesions are more extensive and can involve the small branches of the coronary stirred pulse. Atherosclerotic lesions mainly affect the inner membrane of the coronary stirred pulse. In the early stages of the disease, lipids and lipid-laden macrophages infiltrate the inner membrane and middle layer cells, and the inner membrane thickens, showing yellow spots. As the inner membrane cells are injured and the permeability of the inner membrane increases due to various reasons, lipid infiltration increases, and the spots gradually increase and expand, forming patches or streaks. The inner membrane also shows focal dense layered collagen, and when the lesions involve the entire circumference of the inner membrane, it leads to narrowing or obstruction of the vascular lumen. The blood flow of the diseased coronary stirred pulse decreases, and the local myocardial blood and oxygen supply are insufficient during exercise or even at rest, which can lead to myocardial infarction in severe cases. Atherosclerotic lesions of the coronary stirred pulse can be complicated by hemorrhage, thrombosis, and stirred pulse aneurysm. When atherosclerotic lesions rupture and bleed, lipids enter the vascular lumen, easily causing distal vascular embolism and inducing thrombosis. The hematoma in the vascular wall can gradually form granulation tissue and fibrosis. Acute hemorrhage in the inner membrane may promote spasm of the coronary stirred pulse and collateral circulation branches, exacerbating the degree of myocardial ischemia. Thrombosis often coexists with hemorrhage and can also lead to distal vascular embolism and vascular wall fibrosis. Atherosclerotic lesions of the inner membrane of the coronary stirred pulse with dissipating ecchymosis and necrosis of the middle layer of the vascular wall complicated by stirred pulse aneurysm are very rare. Most cases have only one site of vascular aneurysm, which can reach a diameter of 2.5cm, and the lumen may contain blood clots, but the vascular lumen remains patent. If the coronary stirred pulse stenosis caused by atherosclerotic lesions is limited to one branch of the coronary stirred pulse and the progression is slow, the collateral circulation between the diseased vessel and the adjacent coronary stirred pulse significantly expands, establishing an effective collateral circulation, and the affected myocardial region can still receive sufficient blood supply. If the lesions involve multiple vessels, or the stenosis progresses rapidly, and the collateral circulation is not fully established or is complicated by hemorrhage, hematoma, thrombosis, vascular wall spasm, etc., it can lead to severe myocardial ischemia or even myocardial infarction. The myocardial tissue in the affected area atrophies or even necroses, leading to rupture or later formation of fibrous scars, severely impairing myocardial contractile function, which can result in arrhythmias or heart pump failure. The larger the range of myocardial ischemia, the more severe the damage. The left coronary stirred pulse supplies the most coronary circulation blood flow, so the heart lesions caused by obstruction of the left coronary stirred pulse and its branches are more severe than those of the right coronary stirred pulse.
Pathophysiology: The blood flow to the myocardium is 60-80 ml per 100 g per minute, which is about 10 times more than the blood flow to the whole body tissue, which is 7 ml per 100 g per minute. Another characteristic of coronary circulation is that the blood flow is highest during the diastolic period stirred pulse, while during the systolic period, due to the compression of myocardial vessels, the coronary blood flow decreases, whereas in other organs of the body, the blood flow is highest during the systolic period when the stirred pulse perfusion pressure is highest. The myocardium has a strong oxygen uptake capacity, capable of extracting about 65-75% of oxygen from the capillaries. Under normal conditions, the myocardium extracts 8-10 ml of oxygen per 100 g per minute, while the whole body organs and tissues can only extract 25% of oxygen from the blood, with only about 0.3 ml of oxygen extracted per 100 g of tissue per minute. During exercise, cardiac output significantly increases, the workload of the heart increases, and myocardial oxygen demand increases. Since there is little room to further increase oxygen extraction from the blood, it is necessary to expand the coronary stirred pulse lumen and increase coronary blood flow to meet the increased oxygen demand. The coronary circulation has a sensitive regulatory capacity, and the factors regulating coronary blood flow include: stirred pulse perfusion pressure, coronary vascular resistance, heart rate, duration of cardiac systole and diastole, blood CO2 tension, O2 tension, pH, and neurohumoral factors.
The fundamental substances for myocardial energy metabolism include glucose, fatty acids, lactate, etc. In cases of insufficient coronary blood supply, where the myocardium is in a state of hypoxic metabolism, the oxidation of fatty acids decreases, and the oxidation of carbohydrates becomes predominant. However, under hypoxic conditions, the energy supplied by the breakdown of glucose and glycogen is only a small fraction of that under aerobic metabolism. Continuous myocardial ischemia and hypoxia for more than 20 minutes can cause irreversible mitochondrial damage, myocardial cell necrosis, and loss of myocardial enzyme activity, clinically presenting symptoms such as colicky pain, arrhythmias, and heart failure.
bubble_chart Clinical Manifestations
Symptoms: The main symptom of coronary atherosclerosis heart disease is myocardial ischemia caused by an imbalance between oxygen supply and demand, leading to transient ischemic colicky pain, which often occurs suddenly during exertion, emotional excitement, overeating, or exposure to cold. Common pain locations include behind the sternum or in the precordial area, which may radiate to the inner side of the left arm, shoulder, interscapular region, neck, throat, and jaw, and sometimes in the upper abdomen. The nature of the pain can be severe colicky pain, squeezing pain, pressing pain, tightness, or mild discomfort with a feeling of bloating. Occasionally, severe pain may be accompanied by sweating and a sense of impending doom. The pain usually lasts for 1 to 10 minutes and disappears after rest or taking nitroglycerin tablets. When the cause, frequency, and duration of the colicky pain are relatively stable, it is called stable angina. In some cases, the degree of myocardial ischemia is more severe, and it can transition from typical stable angina to unstable angina, characterized by frequent episodes of colicky pain, prolonged pain duration, increased severity, or even pain at rest, increasing the risk of acute myocardial infarction. In the early stages of acute myocardial infarction, there may be nausea, vomiting, hiccups, or upper abdominal distension and fullness, with severe colicky pain lasting for several hours, not relieved by rest or nitroglycerin tablets, often accompanied by shock, arrhythmias, and heart failure.
Signs: Patients with coronary atherosclerosis heart disease often show no special signs at usual times. During an episode of colicky pain, blood pressure may slightly increase or decrease, and heart rate may be normal, accelerated, or slowed. Severe pain may cause anxiety, dysphoria, pale skin, sweating, and occasionally atrial or ventricular gallop. In cases with papillary muscle dysfunction, a systolic murmur may be heard in the apical region. In myocardial infarction cases, heart rate may increase or decrease, blood pressure may drop, the area of cardiac dullness may slightly enlarge, the first heart sound at the apex may weaken, and sometimes the third or fourth heart sounds or diastolic gallop may appear, along with various arrhythmias, shock, or signs of heart failure.
bubble_chart Auxiliary Examination
X-ray examination: Chest X-ray examination generally shows no abnormalities. In cases accompanied by hypertension, it may reveal left ventricular enlargement, widened, enlarged, and tortuous main stirred pulse. In cases complicated by heart failure, the heart is significantly enlarged, and there is pulmonary congestion.
Electrocardiogram (ECG) examination: ECG is one of the important methods to reflect myocardial ischemia. During episodes of colicky pain, it often shows ST-segment depression, T-wave flattening or inversion. It gradually recovers within a few minutes after the episode, sometimes accompanied by arrhythmias. Patients with no significant abnormalities in routine ECG can undergo stress tests to increase cardiac load and myocardial oxygen consumption, temporarily inducing electrophysiological changes of myocardial hypoxia. ECG stress tests can include double two-step exercise test, treadmill exercise test, bicycle exercise test, and glucose load test. Holter monitoring can also be used for continuous dynamic ECG recording. The ECG characteristics of acute myocardial infarction include deep Q waves or QS waves, significant ST-segment elevation with upward convexity, and T-wave inversion. The location of myocardial infarction can be diagnosed based on the leads showing these characteristic changes.
Serum enzyme tests: In the early stages of acute myocardial infarction, serum aspartate aminotransferase, creatine phosphokinase, and lactate dehydrogenase levels are elevated, and their dynamic changes help assess the progression of the condition.
Other diagnostic methods include echocardiography and radionuclide cardiac imaging, which are valuable for diagnosing coronary heart disease and myocardial infarction, as well as understanding left ventricular function.
Selective coronary stirred pulse angiography and left ventriculography: Selective coronary stirred pulse angiography can clearly visualize the left and right coronary stirred pulse and their branches, providing evidence for the diagnosis of coronary artery stenosis caused by atherosclerotic lesions. It also allows observation of the exact location, extent, degree of stenosis, and collateral circulation of the affected vessels. A reduction of 1/3 in the internal diameter of the affected coronary stirred pulse branch results in a 50% reduction in the vascular lumen area; a reduction of 1/2 in the internal diameter results in a 75% reduction in the lumen area; and a reduction of 2/3 in the internal diameter results in a 90% reduction in the lumen area. Left ventriculography can assess the contraction function of various parts of the left ventricular wall, whether it is normal, decreased, or absent, and measure the left ventricular ejection fraction. Left ventriculography can also diagnose ventricular aneurysm, ventricular septal defect, and mitral regurgitation caused by myocardial infarction. For coronary heart disease cases considered for surgical treatment, selective coronary stirred pulse angiography and left ventriculography are essential preoperatively to clarify surgical indications and formulate a surgical plan.
bubble_chart Treatment Measures
The prevention and treatment methods for coronary heart disease can be broadly categorized into two major types: medical and surgical. Medical treatment has a long history, with measures including dietary and lifestyle adjustments, attention to mental health, the use of drugs to lower blood lipid levels, inhibit platelet aggregation, and control angina pectoris. The surgical treatment of coronary heart disease has also evolved over more than 70 years. In 1916, Jonnesco removed cervical and thoracic sympathetic nerves to treat angina pectoris. In 1926, Boas performed a total thyroidectomy in an attempt to reduce the load on ischemic myocardium by lowering metabolism. In 1935, Beck and Tichy performed pectoralis major and myocardial fixation sutures, hoping that the resulting adhesions would supply blood to the myocardium. Subsequently, tissues and organs such as the pericardium, greater omentum, lung, jejunum, stomach, and spleen were used for myocardial fixation sutures. Some surgeons also used talc powder, asbestos powder, etc., sprinkled in the pericardial cavity to promote pericardial adhesion formation. In 1939, Zola and Cesa-Bianchi ligated the bilateral internal thoracic arteries, believing that the pericardial diaphragmatic arteries near the ligation site could deliver more blood flow to the myocardium. In 1946, Vineberg implanted the internal thoracic artery into the myocardium. In 1955, Beck advocated partial ligation of the coronary sinus and systemic artery branch coronary vein shunt plus partial ligation of the coronary sinus for retrograde perfusion of the coronary circulation. The above various surgical treatment methods were not satisfactory and were gradually abandoned. Starting in 1955, research began on directly performing surgery on the coronary arteries to improve myocardial blood supply. In 1958, Longmire et al. performed endarterectomy on the diseased segment of the coronary artery to relieve lumen stenosis. In 1961, Senning used a graft to repair and enlarge the narrowed segment of the coronary artery under extracorporeal circulation. In 1967, Gatrett performed a left anterior descending branch shunt transplant using the great saphenous vein, which remained patent after 7 years of follow-up. The widespread clinical application of selective coronary angiography rapidly promoted the development of surgical treatment for coronary heart disease. In 1967, Favaloro and Effler popularized the use of the great saphenous vein for ascending aorta-coronary artery bypass grafting and introduced the technique in 1969, performing 741 surgeries by 1971. In 1968, Green reported the internal thoracic artery-coronary artery anterior descending branch anastomosis. In 1971, Flemma et al. reported the sequential grafting technique, using a single great saphenous vein to create multiple anastomoses with several branches of the coronary artery. Since then, surgical treatment for coronary heart disease has entered a new phase. Currently, over 400,000 vascular transplant shunts have been performed worldwide for coronary heart disease, making it the primary surgical treatment method. In 1979, Grüntzig et al. reported percutaneous transluminal coronary angioplasty, a simpler procedure that does not require thoracotomy and has lower medical costs, but with a restenosis rate of 30-40% within 6-9 months post-surgery. In recent years, new techniques and equipment have emerged, such as percutaneous intracoronary thrombolysis for early myocardial infarction caused by coronary artery embolism, and intracoronary cold laser ablation to remove atherosclerotic plaques and stenotic lesions.
Due to the complex factors causing coronary artery atherosclerosis heart disease, it has not yet been fully understood; the number and extent of coronary artery branches affected, the speed of disease progression, and the damage to ventricular function vary greatly. Therefore, a detailed comparison of the effects of medical and surgical treatments based on the natural course of various types of coronary heart disease still requires long-term investigation and research to gradually enrich. According to existing clinical experience, although surgical treatment of coronary heart disease cannot change or reverse the progression of coronary artery atherosclerosis, it can increase coronary artery blood flow and improve coronary circulation. Clinical follow-up data from a large number of cases after ascending aorta-coronary artery bypass grafting using the great saphenous vein indicate good therapeutic effects on angina pectoris, with 60-95% of cases experiencing relief from angina pectoris within 1-5 years post-surgery. Electrocardiograms return to normal, but after 10 years, due to graft occlusion or progression of coronary heart disease, the rate of angina relief decreases to 46%, compared to only 3% in cases without surgical treatment. Physical activity endurance significantly improves in the 3-10 years post-surgery compared to non-surgical cases. Two years post-surgery, 60% of patients can resume normal work, while only 26% of medically treated cases return to work.
Indications for surgical treatment of coronary artery atherosclerotic stenosis: The use of the great saphenous vein to perform ascending aorta-coronary artery bypass grafting, commonly known as bypass surgery, is the most commonly used surgical method for the treatment of coronary heart disease. The surgical indications include:
1. Stable angina pectoris. Factors affecting the course and prognosis of stable angina pectoris include: the number of coronary artery branch lesions, especially whether the left main coronary artery or the anterior descending branch is involved, the functional status of the left ventricle, the severity of myocardial ischemia, the patient's gender and age, and whether there are other diseases. For one or two coronary artery occlusive lesions that do not involve the left main coronary artery, the long-term efficacy of medical treatment is similar to that of surgical treatment. Medical treatment should be initiated first, with regular follow-up. However, if chronic stable angina pectoris does not respond to medical treatment with nitrates, β-blockers, calcium antagonists, and other anti-angina drugs, and the patient's work and life are severely affected, selective coronary angiography should be performed. If the vascular lumen area is reduced by more than 50%, especially if the lesion involves the left main coronary artery, the left anterior descending branch, or three branches of the coronary artery, surgical treatment should be considered.
2. Unstable angina pectoris. In the vast majority of cases, the degree of coronary artery occlusion is severe. Some cases already have small or scattered subendocardial myocardial infarctions and may develop into acute myocardial infarction, severe arrhythmias, or sudden death in a short period. Gazes et al. reported 1-year, 2-year, and 10-year mortality rates of 18%, 25%, and 50%, respectively. If angina pectoris is not controlled after 1 week of aggressive medical treatment in such cases, selective coronary angiography should be performed, and surgical treatment should be considered as soon as possible based on the results.
3. Acute myocardial infarction cases. There is still no consensus on performing coronary artery bypass grafting. Supporters of surgical treatment believe that performing surgery within 8 hours of myocardial infarction can reduce the area of myocardial infarction, resulting in less myocardial scar tissue formation, and lower incidence of complications such as left ventricular aneurysm, arrhythmia, heart failure, and sudden death. The improvement in left ventricular function is also more significant. However, the surgical mortality rate for coronary artery bypass grafting in acute myocardial infarction cases is high, and the incidence of postoperative complications is also high. Long-term follow-up data on efficacy are still lacking. However, for cases with significant ST-segment depression on the treadmill stress test 2 weeks after myocardial infarction, the 1-year mortality rate is 13 times higher than those with negative tests. Surgical treatment should be considered for such cases.
In recent years, thrombolysis and percutaneous transluminal coronary angioplasty have been used to treat early myocardial infarction cases. The long-term efficacy of these treatments and their comparison with bypass grafting are still lacking sufficient data, and no conclusions can be drawn.
4. Severe ventricular arrhythmias, post-myocardial infarction convalescence, or advanced stage cases presenting with severe ventricular arrhythmias. According to statistics, about 1/3 to 1/2 of these cases experience sudden death within 2 to 3 years of follow-up. Therefore, ischemic ventricular arrhythmias should be considered as an indication for coronary artery bypass grafting.
Surgical techniques for coronary artery disease: Surgical procedures to reconstruct coronary blood flow can utilize the great saphenous vein or the internal thoracic artery. The great saphenous vein has a large diameter and is easily accessible, making it the most commonly used in the highest number of cases, with relatively satisfactory outcomes. In recent years, there has been an increasing number of cases using the pedicled internal thoracic artery for end-to-side anastomosis with the coronary artery, particularly the left anterior descending branch. The internal thoracic artery rarely develops atherosclerotic lesions, and postoperative narrowing of the vessel lumen due to intimal hyperplasia is also very rare. The patency rate of the internal thoracic artery after surgery is higher than that of the great saphenous vein, but the dissection and mobilization of the internal thoracic artery are more technically challenging, with a higher incidence of postoperative bleeding complications. Mobilizing both internal thoracic arteries may adversely affect sternal healing. Selective coronary angiography showing coronary artery branches larger than 1.5mm with a lumen diameter reduction of more than 50% should undergo bypass surgery to fully restore myocardial blood flow. In cases of multi-vessel coronary artery disease, bypass surgery may sometimes be required for five or more branches. To simplify the surgical procedure and shorten the operation time, sequential anastomosis can be used, which involves using a segment of the great saphenous vein to perform one or two side-to-side anastomoses proximal to the end-to-side anastomosis. This allows a single vein segment to bypass two or more coronary arteries, reducing the number of anastomoses between the main artery and the great saphenous vein. Sequential anastomosis results in a higher vessel patency rate and faster blood flow, but the procedure requires meticulous and accurate technique, taking care to avoid twisting of the great saphenous vein. Sometimes, a Y-shaped anastomosis can be performed using a thicker branch of the great saphenous vein with another coronary artery branch.
Preoperative preparation: Preoperative examination of lung, liver, and kidney function is required. Stop taking Rehmannia and diuretics 2 days before surgery, but there is no need to stop taking nitroglycerin, β-blockers, and calcium channel blockers.
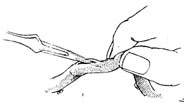
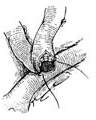
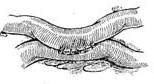
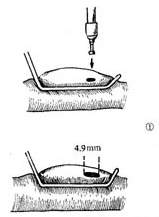
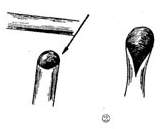
Great Saphenous Vein Bypass Graft Surgery Technique: Endotracheal intubation general anesthesia, surgery performed with extracorporeal circulation combined with low warm purgation, intraoperative monitoring of blood pressure, central venous pressure, electrocardiogram, body temperature, urine output, etc. Skin preparation should include the chest, abdomen, groin, and both lower limbs. The surgical team is divided into two groups to simultaneously perform thoracotomy and harvesting of the great saphenous vein. When freeing the great saphenous vein, the operation must be gentle, avoiding pulling the vein to cause injury. Using a long incision on the lower limb to free the vein is better than using multiple small incisions, as it better protects the vessel from traction injury. During the process of freeing the great saphenous vein, avoid using vascular clamps to clamp the vein; when using tissue forceps, only the outer membrane of the vein wall should be held to prevent injury to the inner membrane. When handling venous branches, care should also be taken to cut and ligate the branches at a distance from the main vein to avoid causing the vein wall to shrink, leading to a reduction in the vascular lumen. The lower segment of the great saphenous vein near the ankle is more suitable for bypass grafting due to the absence of venous valves and better pressure resistance compared to the upper segment. After removing a segment of the great saphenous vein, a smooth needle-tipped syringe is inserted and ligated at the distal end to identify the proximal and distal ends of the vein, and a small amount of cold heparin solution (10,000 units of heparin in 1000ml solution) can be injected to expand the vascular lumen and check for any leaks or fistulas. The removed vein is stored in a 10℃ solution with moderate expansion after injecting the solution into the lumen. Local use of a dilute concentration of papaverine (60mg per 500ml of saline) can prevent venous spasm. While freeing and harvesting the great saphenous vein, another group of surgeons makes a median sternotomy incision, longitudinally splits the sternum, opens the pericardium, exposes the heart, inserts drainage and infusion catheters into the vena cava and the distal segment of the ascending aorta, places a decompression drainage catheter in the left heart chamber, connects to the heart-lung machine, and establishes extracorporeal circulation. During the surgery, myocardial protection should be emphasized, using blood cooling, local myocardial cooling, and perfusion of cold cardioplegic solution, and the time of blocking the ascending aorta should be minimized as much as possible. Generally, the distal great saphenous vein is anastomosed to the coronary artery branch first, but sometimes the ascending aorta is anastomosed to the great saphenous vein before the distal anastomosis. At the selected anastomosis site, the coronary artery branch is exposed, the anterior wall of the artery is longitudinally incised with a sharp knife, and then the coronary artery incision is enlarged to about 6-8mm with fine curved scissors. Sometimes a small triangular piece of the anterior wall of the coronary artery branch is removed to facilitate the vascular anastomosis. The end of the great saphenous vein for anastomosis is trimmed and cut at a 45° angle to prevent twisting after the anastomosis is completed. If necessary, a small segment of the vein wall can be longitudinally cut to enlarge the anastomosis end, and the length of the great saphenous vein incision should be 10-20% longer than the coronary artery branch incision. The anastomosis is sutured with 6-0 or 7-0 Prolene suture in a continuous or interrupted manner, with a stitch distance of about 1mm, slightly wider on the great saphenous vein side, ensuring proper alignment of the inner membrane. During the anastomosis process, a small amount of heparin solution is dripped into the other end of the great saphenous vein to help improve the surgical field exposure. For sequential anastomosis, after completing the most distal end-to-side anastomosis, according to the direction of the transplanted great saphenous vein and its anatomical relationship with another coronary artery branch, a longitudinal or transverse incision is made on the great saphenous vein wall for anastomosis. It is essential to ensure that the distance between several incisions on the great saphenous vein is appropriate, the anastomosis does not twist, and blood flow is smooth. After completing the distal anastomosis, the aortic clamp can be released, and extracorporeal circulation rewarming can begin.With the great saphenous vein kept distended, select the ascending aorta anastomosis site, partially clamp the aortic wall with a non-traumatic vascular clamp, and create a small hole approximately 5mm in diameter at each anastomosis site using an aortic wall perforator. Obliquely cut the end of the great saphenous vein to be anastomosed, ensuring the venous anastomosis is 10-20% larger than the aortic wall incision. Use 5-0 Prolene suture to continuously suture the anastomosis (Figure 1).
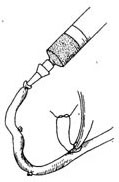
(1) Expand the venous lumen with heparin solution
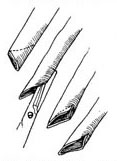
(2) Cut the end of the great saphenous vein at a 45° angle, then make a longitudinal incision
(3) End-to-side anastomosis of the great saphenous vein-coronary stirred pulse
 |  |
 |  |
 |  |
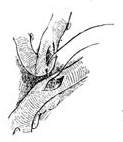
(4) Sequential anastomosis: The incision in the great saphenous vein can be longitudinal or transverse
① Ascending aorta stirred pulse
② Great saphenous vein
(5) End-to-side anastomosis of the great saphenous vein-ascending aorta stirred pulse
Figure 1: Using the great saphenous vein for main stirred pulse-coronary stirred pulse bypass grafting
End-to-side anastomosis of the internal thoracic stirred pulse-coronary stirred pulse: The diameter of the internal thoracic stirred pulse is similar to that of the coronary stirred pulse. The patency rate of the internal thoracic stirred pulse after performing a shunt procedure is higher than that of the great saphenous vein, and there is no need to perform a proximal main stirred pulse anastomosis. However, due to its limited length and anatomical position, it is generally only suitable for anastomosis with the left anterior descending branch or diagonal branch of the left coronary artery using the left internal thoracic stirred pulse. The diameter of the right internal thoracic stirred pulse is smaller than that of the right coronary stirred pulse, and its length is insufficient, so it is rarely used for anastomosis with the branches of the right coronary stirred pulse.
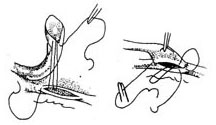
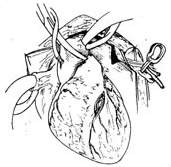
After splitting and spreading the sternum, before incising the pericardium and injecting heparin, use an electric knife to cut the internal thoracic fascia along both sides of the internal thoracic artery, about 1 cm away from the vessel, starting from the 6th intercostal space upwards to above the sternum. Free the internal thoracic artery, vein, surrounding adipose tissue, muscle, and thoracic fascia. The branches of the intercostal artery need to be ligated and cut. The freed internal thoracic artery and its surrounding tissues should be carefully protected from trauma and wrapped with gauze soaked in a dilute solution of papaverine. After systemic heparinization before starting extracorporeal circulation, ligate and cut the internal thoracic artery at the level of the 6th intercostal space. The bleeding from the proximal end of the artery can reach 120-240 ml per minute. If the bleeding is less than 100 ml per minute, the vessel quality is poor and should not be used. Incise the left anterior descending artery, select an appropriate length to remove the surrounding tissue of the distal segment of the internal thoracic artery, exposing about 1 cm of the artery. The lumen can be gently expanded with a dilator of 1.0 to 1.5 mm in diameter and then obliquely cut. Use 7-0 Prolene suture to perform continuous or interrupted sutures with the incision of the anterior descending artery. Then, use several interrupted sutures to fix the surrounding soft tissue of the internal thoracic artery to the myocardium to reduce the tension at the anastomosis site. Transversely incise the left pericardium to ensure an unobstructed pathway for the internal thoracic artery into the heart (Figure 2).
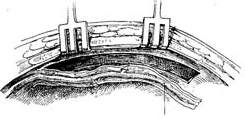
(1) Free the left internal thoracic stirred pulse
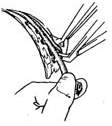
(2) Ligate and cut the intercostal stirred pulse branches
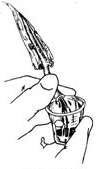
(3) Measure the amount of bleeding
(4) Incise the anterior descending branch and pericardial membrane

(5) Vascular anastomosis
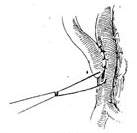
(6) Anastomosis nearing completion
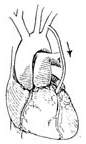
(7) Schematic diagram of blood flow after anastomosis completion
Figure 2: Internal thoracic stirred pulse-coronary stirred pulse anastomosis
When performing coronary stirred pulse bypass grafting, if the condition of the lesion requires, a coronary stirred pulse endarterectomy can be performed simultaneously.
Postoperative management: After coronary stirred pulse bypass grafting, closely monitor blood pressure, central venous pressure, left atrial pressure, heart rate, rhythm, body temperature, thoracic drainage volume, urine output, blood gas analysis, blood pH, and electrolyte levels to prevent hypovolemia, hypoxia, acidosis, and electrolyte imbalance. Oral β-blockers can prevent and treat arrhythmias. For cases where the great saphenous vein is used for bypass grafting, postoperative administration of aspirin and dipyridamole can prevent deep vein thrombosis in the lower limbs. A small number of patients may develop low cardiac output syndrome postoperatively, and if drug treatment is unsatisfactory, intra-aortic balloon counterpulsation may be required.
Efficacy of bypass grafting: In recent years, the surgical mortality rate has dropped to below 5%. The most common cause of death is acute heart failure. Factors affecting surgical mortality include the extent of coronary stirred pulse branch lesions, the severity of angina, preoperative left ventricular function, patient age, gender, whether myocardial infarction is complicated, the number of graft vessels, aortic cross-clamp time, and the adequacy of surgical technique. Perioperative myocardial infarction is a common factor affecting efficacy, with an incidence rate of 2-10%. Mild cases may only show abnormal serum enzyme levels, while severe cases may present with ECG changes. Improving anesthesia techniques and emphasizing myocardial protection during surgery can reduce the incidence of myocardial infarction.
Long-term efficacy of bypass grafting: After bypass surgery, angina is significantly reduced or disappears, left ventricular function improves, cardiac output increases, and heart function significantly improves. About two-thirds of patients can return to work postoperatively, and nearly 70% of cases can survive for more than 10 years. The 10-year survival rate for single-vessel coronary stirred pulse disease is 78%, for two-vessel disease is 69%, for three-vessel disease is 48%, and for left main coronary stirred pulse disease is 67%. After bypass grafting, intimal hyperplasia in the great saphenous vein may occur, leading to vessel narrowing and poor blood flow. The incidence of varying degrees of intimal hyperplasia in the great saphenous vein within 5 years postoperatively can reach 10-45%.




