| disease | Thoracic Aortic Arch Aneurysm |
Aortic arch aneurysms are relatively rare. Since the lesion is located at the origin of the brachiocephalic artery branch of the aortic arch, the surgical procedure is relatively complex. During the operation, it is essential to maintain blood flow perfusion to the brain and heart to avoid ischemic and hypoxic damage.
bubble_chart Etiology
The most common cause of aortic arch aneurysms is atherosclerosis. Other causes include cystic medial necrosis, trauma, and infection, while syphilitic aortitis-induced aneurysms are rare. As the aneurysm enlarges, it can compress adjacent mediastinal structures such as the superior vena cava, innominate vein, pulmonary artery, trachea, bronchi, lungs, and left recurrent laryngeal nerve. If the aneurysm ruptures into the pulmonary artery or systemic venous circulation, an arteriovenous fistula may form. Due to the large shunt volume, this can lead to heart failure and death. Aortic arch aneurysms may also rupture into the pericardial cavity, pleural cavity, trachea, or bronchi, causing acute cardiac tamponade or fatal hemorrhage.
bubble_chart Clinical Manifestations
Aortic aneurysm. Aortic aneurysm. The compression of adjacent mediastinal organs and tissues by the tumor can cause symptoms such as dyspnea, wheezing, cough, hemoptysis, chest pain, and hoarseness. Compression of the superior vena cava results in distension of the veins in the head, face, and upper limbs, while compression of the left innominate vein leads to distension and enlargement of the left upper limb and left jugular vein, with venous pressure in the left upper limb higher than that in the right. Physical examination may reveal abnormal pulsations in the upper anterior chest and cardiac murmurs. Left vocal cord paralysis may occur, and signs of congestive heart failure may sometimes be present. Chest X-ray shows a pulsatile mass in the upper mediastinum, and computed tomography helps determine whether the aortic aneurysm contains thrombus. Aortic angiography not only visualizes the aortic aneurysm and confirms the diagnosis but also delineates the extent of the aneurysm and whether the brachiocephalic artery branches are involved. For patients over 50 years old, selective coronary angiography is also required to determine if coronary artery disease is present.
bubble_chart Treatment MeasuresThe treatment principle for aortic arch aneurysm is to resect the aortic arch aneurysm and perform an artificial blood vessel graft to restore normal blood flow in the aorta and its major branches. During the operation, it is essential to protect the heart, brain, spinal cord, and visceral organs from ischemic and hypoxic damage. Specific protective measures include the following methods.
1. Temporary artificial vascular shunt: Surface hypothermia anesthesia is administered, and a midline incision is made in the anterior chest. The sternum is longitudinally sawed open, and the pericardial membrane is incised to determine the extent of the aneurysm proximally and distally. The proximal and distal ascending and descending aorta are dissected free. Systemic heparinization is performed. The ascending and descending aortic walls are partially clamped sequentially, and end-to-side anastomoses are performed with a pre-clotted artificial vascular segment. A bifurcated artificial blood vessel is then sutured to the main artificial vessel, and the two branches are anastomosed end-to-side to the left and right common carotid arteries. This allows blood to flow from the ascending aorta into the descending aorta and both common carotid arteries via the artificial vessel when the aortic arch blood flow is blocked. Non-traumatic vascular clamps are placed between the anastomotic sites of the artificial vessel and the ascending/descending aorta and the aneurysm to block the aneurysm's blood flow. Clamps are also placed at the roots of the innominate artery, left common carotid artery, and left subclavian artery. After resecting the aneurysm, another segment of an artificial vessel of appropriate length and diameter is used to replace the aortic arch. Both ends of the artificial vessel are anastomosed end-to-end with the cut ends of the ascending and descending aorta. The cut ends of the innominate artery, common carotid artery, and left subclavian artery are anastomosed end-to-side to incisions on the upper wall of the artificial vessel. After completing the aortic arch replacement, the descending aortic clamp is removed first to expel any residual air in the artificial vessel. Then, the clamps on the ascending aorta, innominate artery, left common carotid artery, and left subclavian artery are removed to restore aortic arch blood flow. Finally, the temporary shunt artificial vessel is dismantled, and the incisions in the ascending/descending aorta and both carotid arteries are sutured (Figure 1).
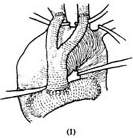 | 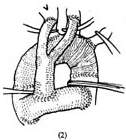 | 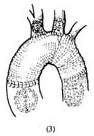 |
Figure 1: Resection of aortic arch aneurysm and artificial blood vessel graft
(1) First, an end-to-side anastomosis is performed between the ascending and descending aorta using a pre-clotted artificial vascular segment. A bifurcated artificial blood vessel is sutured to the main artificial vessel, and its two branches are anastomosed end-to-side to both common carotid arteries. Then, the vascular clamps are applied proximal and distal to the aneurysm.
(2) The aortic arch aneurysm is resected, and the artificial vessel is implanted. The vascular clamps are removed to restore aortic blood flow. Finally, the temporary shunt artificial vessel is dismantled, and the incisions in the ascending/descending aorta and both common carotid arteries are sutured.
(3) The resection of the aortic arch aneurysm is completed.
In 1957, DeBakey successfully used an artificial vascular temporary shunt to replace the aortic arch. This method is suitable for cases where aortic arch tumor diseases are limited to the aortic arch, while the ascending aorta and proximal descending aorta have normal vessel walls, facilitating end-to-side anastomosis with the artificial vessel. This technique does not require the use of extracorporeal circulation, but its main drawback is the need to perform multiple anastomoses. Some anastomoses require suturing after removal, making the surgical procedure difficult and complex, with a prolonged operation time. Additionally, there is an increased risk of postoperative bleeding at the anastomotic or sutured sites. Currently, this method is rarely used.
2. Extracorporeal circulation combined with separate perfusion of the brachiocephalic stirred pulse branches and coronary stirred pulse The operation is performed under systemic extracorporeal circulation combined with moderate hypothermic purgation. Blood drainage catheters are placed in the superior and inferior vena cava through incisions in the right atrium and right auricle, or a single drainage catheter is placed in the right atrium. A decompression catheter is placed in the left atrium. Blood infusion catheters are inserted into the femoral stirred pulse, right subclavian stirred pulse, left common carotid stirred pulse, left subclavian stirred pulse, and coronary stirred pulse. To ensure appropriate perfusion pressure and flow in the brachiocephalic stirred pulse branches and coronary stirred pulse, each infusion catheter should be equipped with its own blood pump, with a perfusion flow rate of approximately 500 ml per minute per catheter (Figure 2). After clamping the ascending and descending aorta and the brachiocephalic stirred pulse branches, the aortic arch stirred pulse aneurysm is resected, and a segment of artificial blood vessel is used to replace the aortic arch. The two ends of the artificial blood vessel are anastomosed end-to-end with the ascending and descending aorta, respectively. To simplify the procedure and reduce the number of anastomoses, the origins of the three brachiocephalic stirred pulse branches, along with the adjacent wall of the ascending aorta, can be excised from the upper wall of the ascending aorta and anastomosed to the corresponding incision on the artificial blood vessel (Figure 3).
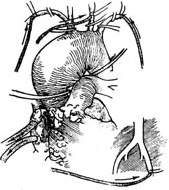
Figure 2. Extracorporeal circulation combined with separate perfusion of the brachiocephalic stirred pulse and coronary stirred pulse
Under systemic extracorporeal circulation, blood drainage catheters are placed in the superior and inferior vena cava through incisions in the right atrium and right auricle. A decompression catheter is placed in the left atrium, and blood infusion catheters are inserted into the femoral stirred pulse, right subclavian stirred pulse, left common carotid stirred pulse, left subclavian stirred pulse, and coronary stirred pulse. Then, after clamping the ascending and descending aorta and the brachiocephalic stirred pulse branches, the aortic stirred pulse aneurysm is resected, and an artificial blood vessel is transplanted.
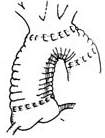
Figure 3. Anastomosis of the three brachiocephalic stirred pulse branches to the upper wall of the artificial blood vessel
3. Extracorporeal circulation combined with deep hypothermia and interrupted perfusion A median sternotomy is performed, and the pericardium is incised. Blood drainage catheters are placed in the superior and inferior vena cava through incisions in the right atrium and right auricle, or a single drainage catheter is placed in the right atrium. A decompression and drainage catheter is placed in the left atrium, and a blood infusion catheter is inserted into the femoral stirred pulse. Extracorporeal circulation is initiated, and the body temperature is lowered to approximately 15–20°C nasopharyngeal temperature. Vascular clamps are placed at the proximal ascending aorta and the roots of the brachiocephalic stirred pulse branches to block the chinse clinopodium herb. Cold cardioplegic solution is injected into the root of the ascending aorta. Then, blood infusion through the femoral stirred pulse is stopped, and after about 10 seconds, the venous drainage catheters are clamped. Depending on the specific condition of the aortic arch stirred pulse tumor diseases, the stirred pulse aneurysm is resected, and an artificial blood vessel is transplanted. For stirred pulse tumor diseases limited to the proximal segment and inferior wall of the aortic arch, the stirred pulse aneurysm is resected, and the proximal aortic arch and its inferior wall are replaced with an artificial blood vessel, preserving the superior wall of the aortic arch and the brachiocephalic stirred pulse branches (Figure 4).
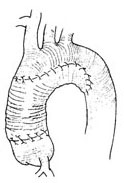
Figure 4. Surgical method for stirred pulse aneurysm limited to the proximal segment and inferior wall of the aortic arch
The superior wall of the aortic arch and the brachiocephalic stirred pulse branches are preserved. After resection of the stirred pulse aneurysm, the proximal aortic arch and its inferior wall are replaced with an artificial blood vessel.
For a saccular stirred pulse aneurysm in the inferior wall of the aortic arch, the stirred pulse aneurysm can be incised to expose the aortic wall rupture, which is then repaired with a patch and reinforced with stirred pulse wall sutures (Figure 5).
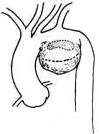 | 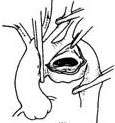 |
| (1) The dotted line indicates the incision for the stirred pulse tumor. | (2) Shows the rupture in the main stirred pulse wall. |
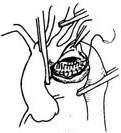 | 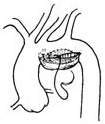 |
| (3) The main stirred pulse rupture is repaired with a Dacron patch. | (4) The main stirred pulse aneurysm wall is reinforced with sutures. |
Figure 5: Surgical method for a sac-like main stirred pulse aneurysm on the lower wall of the stirred pulse arch.
For stirred pulse tumor diseases involving the entire main stirred pulse arch, a total arch replacement is required. After clamping the ascending main stirred pulse and the brachiocephalic stirred pulse branches, to reduce procedural difficulty and shorten surgery time, dissection outside the stirred pulse aneurysm is unnecessary. Instead, a longitudinal incision is made in the middle of the stirred pulse aneurysm to remove the thrombus inside, taking care to prevent thrombus fragments from falling into the descending main stirred pulse. The selected artificial vessel, pre-treated for coagulation, is first anastomosed end-to-end with the descending main stirred pulse within the stirred pulse aneurysm cavity, using 3-0 Prolene sutures with small pledgets for interrupted or continuous suturing. After confirming no fistula disease leakage at the anastomosis, an elliptical window is cut on the upper wall of the artificial vessel for an end-to-side anastomosis with the origin of the brachiocephalic stirred pulse branches and the surrounding upper wall of the main stirred pulse arch (Figure 6). Once the anastomoses of the descending main stirred pulse and brachiocephalic stirred pulse branches with the artificial vessel are completed, the patient is placed in a head-down position. The artificial vessel is clamped near the ascending main stirred pulse end, and blood supply is gradually restored via the femoral stirred pulse while purging residual air from the artificial vessel. The brachiocephalic stirred pulse branch clamps are then released, and extracorporeal circulation rewarming begins. After trimming the other end of the artificial vessel, it is anastomosed end-to-end with the ascending main stirred pulse. The artificial vessel clamp is released, and an air vent needle is inserted into the ascending main stirred pulse to remove air before releasing the ascending main stirred pulse clamp. Multiple anastomoses are checked for fistula disease bleeding, with additional sutures applied if needed. Once the heart beats strongly and body temperature reaches above 35°C, extracorporeal circulation can be stopped. The stirred pulse aneurysm wall is trimmed, and the cut edges are sutured with interrupted stitches to tightly wrap around the artificial vessel. Cardiac and stirred pulse cannulas are removed, and the surgery is concluded following standard procedures. The safe time limit for deep hypothermic circulatory arrest should not exceed 45 minutes.
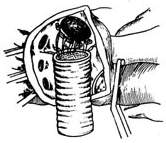
(1) As described earlier, a longitudinal incision is made in the middle of the stirred pulse aneurysm to remove the thrombus, and the pre-treated artificial vessel is anastomosed end-to-end with the descending main stirred pulse within the stirred pulse aneurysm cavity.

(2) An elliptical window is cut on the upper wall of the artificial vessel for an end-to-side anastomosis with the origin of the brachiocephalic stirred pulse branches and the surrounding upper wall of the main stirred pulse arch.
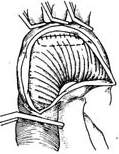
(3) After trimming the other end of the artificial vessel, it is anastomosed end-to-end with the ascending main stirred pulse. The vascular clamps are sequentially removed, and the stirred pulse aneurysm edges are sutured with interrupted stitches to tightly wrap around the artificial vessel.
Figure 6: Resection method for stirred pulse tumor diseases involving the entire main stirred pulse arch.





