| disease | Atherosclerosis |
| alias | Atherosclerosis |
Atherosclerosis is a common type of arterial sclerosis and the main cause of myocardial infarction and cerebral infarction. Arteriosclerosis refers to the degenerative and proliferative changes in the arterial wall, characterized by thickening, hardening, and narrowing of the lumen. The common types include: 1. Atherosclerosis; 2. Medial calcification; 3. Arteriolosclerosis (small artery hardening).
bubble_chart Etiology
The etiology of this disease is not fully understood. It is currently believed that the disease is caused by multiple factors acting on different stages, and these factors are referred to as predisposing or risk factors. The main factors include: ① Age: It is more common in middle-aged and elderly individuals over 40 years old. Progression accelerates after the age of 49, but early lesions can also occur in young adults. ② Gender: It is more prevalent in males, with a male-to-female ratio of 2:1. In females, it is often seen after menopause. ③ Hyperlipidemia: Elevated levels of total cholesterol, low-density lipoprotein (LDL), triglycerides, very low-density lipoprotein (VLDL), apolipoprotein B100, and lipoprotein(a) (Lp(a)), as well as decreased levels of high-density lipoprotein (HDL) and apolipoproteins AⅠ and AⅡ, are all considered predisposing factors. ④ Hypertension: 60–70% of patients with coronary artery atherosclerosis have hypertension. The incidence of coronary artery atherosclerosis in hypertensive patients is four times higher than in those with normal blood pressure, and both systolic and diastolic hypertension are significant. ⑤ Smoking: Smoking increases the incidence and mortality of coronary artery atherosclerosis by 2–6 times, and this effect is proportional to the number of cigarettes smoked daily. ⑥ Diabetes: The incidence of atherosclerosis in diabetic patients is twice as high as in non-diabetic individuals, and impaired glucose tolerance is quite common in patients with coronary artery atherosclerosis.
Less significant factors include: ① Occupation: Those with sedentary jobs, high mental stress, or frequent feelings of urgency are more susceptible to the disease. ② Diet: A diet high in calories, saturated fats, cholesterol, sugar, and salt increases the risk, with Western dietary patterns being a major contributing factor. ③ Obesity: Individuals who are overweight or obese, especially those with rapid weight gain, are more prone to the disease. ④ Type A personality: Traits such as strong ambition, competitiveness, intense focus on work with little relaxation, impatience, and a drive to achieve are associated with a higher risk. ⑤ Trace element intake: Deficiencies in chromium, manganese, zinc, vanadium, and selenium, as well as excesses of lead, cadmium, and cobalt, are risk factors. ⑥ Genetics: Families with a history of the disease at a younger age have a fivefold higher risk among close relatives. Some consider this disease a polygenic inherited cardiovascular disorder. Familial hyperlipidemia caused by autosomal dominant inheritance is often a predisposing factor, and hypertension and diabetes, which are also risk factors, have genetic influences. Other factors that may increase vascular permeability include hypoxia, antigen-antibody complexes, vitamin C deficiency, and reduced enzyme activity in the arterial wall. Deficiencies in vitamins A and E, as well as increased iron storage, are also believed to predispose to the disease. In recent years, cytomegalovirus infection has been suggested as a possible contributor.Over the past half-century, the incidence of this disease has risen significantly in Europe and America, becoming a prevalent condition by the late 1960s. In some countries and regions, heart disease caused by coronary artery atherosclerosis has become the leading cause of death. Since the 1970s, due to increased preventive measures, the incidence has shown a declining trend. Previously, this disease was uncommon in China, but in recent years, with advancements in public health, the control of many diseases, increased life expectancy, and improved living standards, the disease has relatively and absolutely increased, now ranking among the leading causes of death in the population.
The pathogenesis of this disease is not yet fully understood. Although there have been advances in research in recent years, it is still explained from different perspectives through various theories or hypotheses.
(1) The lipid infiltration theory suggests that the disease is closely related to abnormal lipid metabolism, with its essence being the response of the arterial wall to lipids infiltrating from the plasma. The main pathological change in this disease is the appearance of atherosclerotic plaques in the arterial wall, with cholesterol and cholesteryl esters being the primary components of these plaques. Although the arterial wall can also synthesize cholesterol and other lipids, recent studies on the physiology and pathology of the arterial wall and endothelial cells, as well as histochemical and immunochemical examinations of atherosclerotic lesions, have confirmed that the lipids in atherosclerotic plaques mainly originate from the plasma. Plasma cholesterol, triglycerides, and phospholipids are dissolved and transported by binding to apolipoproteins to form lipoproteins. LDL contains the most cholesterol and cholesteryl esters, VLDL contains the most triglycerides, and HDL contains the most protein. Elevated plasma lipids, in the form of LDL and VLDL or their remnants after decomposition by lipoprotein lipase on the surface of the arterial intima, infiltrate the arterial wall through the following pathways: ① direct endocytosis by endothelial cells; ② passage through interendothelial cell gaps; ③ via LDL receptors on endothelial cells; ④ through endothelial cells with increased permeability due to damage; ⑤ through the subendothelial tissue directly exposed to blood flow due to endothelial cell loss. Once lipoproteins enter the media, they accumulate among smooth muscle cells, collagen, and elastic fibers, inducing smooth muscle cell proliferation. Smooth muscle cells and monocytes derived from the blood engulf large amounts of lipids to become foam cells. Lipoproteins also degrade, releasing cholesterol, cholesteryl esters, triglycerides, and other lipids. LDL further binds to arterial wall proteoglycans to form insoluble precipitates, all of which stimulate fibrous tissue proliferation. Together, these processes contribute to the formation of atherosclerotic plaques.
(II) Thrombosis and Platelet Aggregation Theory The former posits that the disease begins with a hyperactive local coagulation mechanism, leading to thrombus formation on the surface of the stirred pulse membrane. Subsequently, the thrombus is covered by proliferating endothelial cells and incorporated into the stirred pulse wall. The disintegration of platelets and white blood cells within the thrombus releases lipids and other active substances, gradually forming an atherosclerotic patch. The latter theory suggests that the disease starts with injury to the stirred pulse membrane, where an increase in platelet-activating factor (PAF) causes platelets to adhere and aggregate at the site, followed by fibrin deposition and the formation of microthrombi. After platelet aggregation, certain active substances are released. Among these, thromboxane A2 (TXA2) counteracts the effects of prostacyclin (PGI2), which is synthesized by the vascular wall and promotes platelet disaggregation and vasodilation, thereby further enhancing platelet aggregation and vasoconstriction. Platelet-derived growth factor (PDGF) stimulates the proliferation and contraction of smooth muscle cells and their migration into the membrane. Serotonin and fibroblast growth factor (FGF) stimulate the proliferation of fibroblasts, smooth muscle cells, and endothelial cells. Epinephrine and adenosine diphosphate (ADP) further promote platelet aggregation. Factor VIII enhances platelet adhesion, while platelet factor 4 (PF4) induces vasoconstriction. Plasminogen activator inhibitor (PAI) inhibits thrombus dissolution. These substances exacerbate endothelial cell injury, facilitating the entry of LDL and fibrinogen into the membrane and subendothelial layer. They also promote the aggregation of monocytes in the membrane, transforming them into foam cells; stimulate smooth muscle cell proliferation and migration into the membrane, where they engulf lipids; and induce endothelial cell proliferation—all of which contribute to the formation of atherosclerosis.
(3) Injury Response Theory Suggests that the formation of atherosclerotic plaques is a reaction to endothelial injury caused by turbulent blood flow. Endothelial injury due to turbulent flow can manifest as endothelial dysfunction, such as increased permeability, making the surface prone to thrombus formation. It may also involve the disruption of endothelial integrity. Chronic hyperlipidemia, along with hemodynamic changes caused by increased blood pressure, specific angles and pathways of turbulent flow branches, and localized vascular narrowing—leading to turbulence and shear stress—as well as the long-term repetitive effects of factors like diabetes, smoking, bacteria, viruses, toxins, immune factors, and vasoactive substances such as catecholamines, serotonin, histamine, kinins, endothelin, and angiotensin, can all injure the endothelium or alter its function. This promotes lipid deposition, platelet adhesion, and aggregation, ultimately leading to atherosclerosis.
(4) Monoclonal Theory Also known as the monoclonal proliferation theory, it posits that each atherosclerotic lesion originates from the proliferation of a single smooth muscle cell, which serves as the progenitor for subsequent cell growth. Under the influence of factors such as platelet-derived growth factor, endothelial-derived growth factor, monocyte-derived growth factor, LDL, and possibly viruses, these cells continuously proliferate and engulf lipids, resembling benign tumors and contributing to atherosclerosis. While studies measuring glucose-6-phosphate dehydrogenase (G6PD) isoenzymes have shown that most atherosclerotic fibrous plaques contain only one G6PD isoenzyme, indicating monoclonal characteristics, some argue that a single-enzyme phenotype does not necessarily prove clonal origin. It could also result from multiple cells sharing the same isoenzyme, with repeated cycles of cell death and growth leading to the observed single-enzyme phenotype. In fact, culturing smooth muscle cells from atherosclerotic plaques has not demonstrated unlimited proliferation akin to tumors.
(5) Other Mechanisms Additional mechanisms related to pathogenesis include neural and endocrine changes, alterations in the quantity and quality of acidic proteoglycans in the arterial wall matrix (increased dermatan sulfate and decreased chondroitin sulfate A and C), and reduced enzymatic activity in the arterial wall. These factors may contribute to atherosclerosis by influencing vascular motility, lipid metabolism, and the anabolic processes of the vessel wall.
bubble_chart Pathological Changes
The pathological changes of atherosclerosis mainly affect the large elastic arteries (such as the aorta) and medium-sized muscular-elastic arteries (with the coronary and cerebral arteries being the most frequently involved, followed by the arteries of the limbs, renal arteries, and mesenteric arteries; the splenic artery may also be affected) in the systemic circulation, while the pulmonary circulation arteries are rarely involved. The lesions are often distributed in multiple tissues and organs simultaneously, but sometimes they may concentrate in the arteries of a specific organ while other arteries remain normal. The earliest lesions typically appear in the posterior wall of the aorta and the openings of intercostal arteries, where blood pressure is higher and the vessel walls endure greater hemodynamic stress, making the lesions more pronounced.
A normal arterial wall consists of three layers: the intima, media, and adventitia (Figure 1). The intima is composed of a single layer of endothelial cells, connective tissue, and a fenestrated internal elastic lamina. Between the endothelial cells and the elastic lamina (also called the subendothelial layer), in addition to connective tissue, there are smooth muscle cells and extracellular matrix (including acidic proteoglycans, soluble proteins, lipids, glucose, and electrolytes). Smooth muscle cells are rare in childhood but gradually increase in number with age, along with the matrix components in the intima. In muscular-elastic arteries, the media is almost entirely composed of obliquely arranged smooth muscle cells, surrounded by varying amounts of collagen, elastic fibers, and glycoproteins, with morphology generally unchanged with age. The adventitia contains fibroblasts, as well as collagen, glycoproteins, and scattered smooth muscle cells. A discontinuous external elastic lamina separates the adventitia from the media. In atherosclerosis, the arterial wall exhibits three types of changes: fatty streaks, fibrous plaques, and complicated lesions.
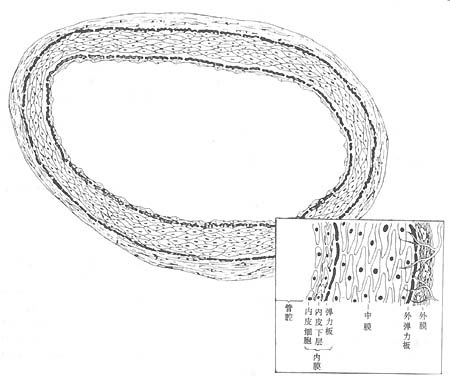
**Figure 1** Schematic structure of the arterial wall
Showing the three layers of the arterial wall—intima, media, and adventitia—with a magnified detail in the lower right corner.
**(1) Fatty streak lesions** These are early-stage lesions, commonly seen in young individuals, and are confined to the arterial intima, appearing as yellow lipid spots a few millimeters in size or yellow fatty streaks up to several centimeters long. The hallmark of these lesions is focal accumulation of macrophages and a few smooth muscle cells in the intima, with intracellular and extracellular lipid deposition. The lipids consist mainly of cholesterol and cholesteryl esters, along with phospholipids and triglycerides. Since fatty streaks are flat or only slightly raised lesions, they do not obstruct the affected artery or cause clinical symptoms. Their significance lies in their potential to develop into plaques.
**(2) Fibrous plaque lesions** These are the most characteristic lesions of progressive atherosclerosis, typically appearing as pale yellow, slightly raised protrusions into the arterial lumen or around the openings of vascular branches, causing luminal narrowing. These lesions mainly consist of proliferated connective tissue in the intima, along with lipid-laden smooth muscle cells and macrophages. The lipids are primarily cholesterol and cholesteryl esters, surrounded by extracellular lipids, collagen, elastic fibers, and glycoproteins. Fibrous tissue proliferation forms a fibrous cap overlying a deeper core of abundant lipids, mixed with cellular debris and cholesterol crystals. As the plaque enlarges, it extends into the media, disrupting the muscular and elastic fibers of the arterial wall, which are replaced by connective tissue and proliferating new capillaries. With significant lipid deposition, the central base of the plaque often undergoes malnutrition-induced degeneration, necrosis, and disintegration, forming a mixture of necrotic debris and lipids known as atheromatous material, giving rise to an atheromatous plaque or atheroma.
(3) Complex combination of diseases changes into fibrous patches, leading to hemorrhage, necrosis, ulcers, calcification, and mural thrombus formation. Atherosclerotic patches may rupture on the inner membrane surface, forming so-called atherosclerotic ulcers. After rupture, the atherosclerotic material enters the bloodstream, becoming emboli. The ruptured site can cause hemorrhage, and the rough ulcer surface is prone to thrombus formation. Mural thrombus formation further aggravates luminal stenosis or even occlusion. As the vessel gradually becomes occluded, collateral circulation from the proximal bleeding vessels gradually develops. After thrombus organization, recanalization may occur, partially restoring local blood flow. Complex combination of diseases also features calcification of the middle membrane (Figures 16-2, 3, 4).
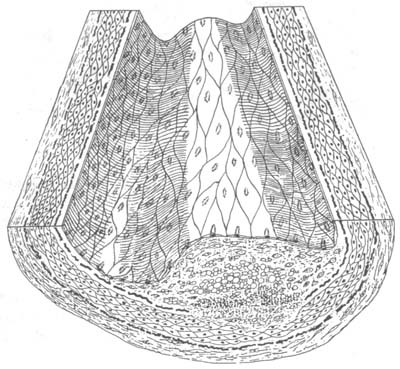
Figure 16-2 Atherosclerosis in stirred pulse
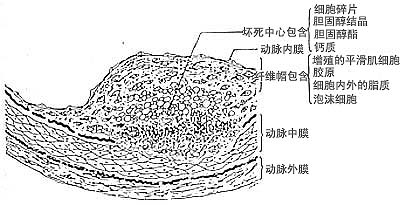
Figure 16-3 Schematic cross-sectional structure of fibrous patch in stirred pulse atherosclerosis
The fibrous patch is shown to be covered by a single layer of endothelial cells, beneath which lies the fibrous cap containing smooth muscle cells, and further below is the necrotic core.
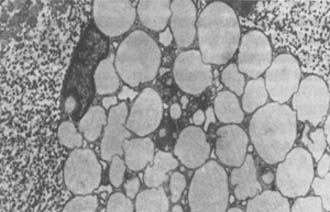
Figure 16-4 Early lesions of stirred pulse atherosclerosis
Transmission electron micrograph showing foam cells in the fatty streak of human stirred pulse, with cytoplasm filled with lipid droplets and nuclei displaced to the periphery (×6500).
The affected stirred pulse exhibits weakened elasticity, increased fragility, and susceptibility to rupture. Its lumen gradually narrows or may become completely occluded, or it may dilate to form a stirred pulse aneurysm.
Depending on the affected stirred pulse and the establishment of collateral circulation, this disease can cause functional disturbances in the entire circulatory system or individual organs:
1. The aorta, due to atherosclerosis, experiences reduced wall elasticity. During cardiac contraction, its temporary expansion and the retention of part of the blood ejected by the heart are weakened, leading to increased systolic pressure and widened pulse pressure. When an atherosclerotic stirred pulse aneurysm forms in the aorta, the wall is replaced by fibrous tissue, losing tension and bulging outward. These factors significantly affect systemic blood flow regulation and increase cardiac workload.
2. Narrowing or occlusion of visceral or limb stirred pulse lumens, when collateral circulation cannot compensate, results in impaired blood supply to organs and tissues, causing ischemia, fibrosis, or necrosis. For example, coronary stirred pulse atherosclerosis can lead to angina pectoris, myocardial infarction, or myocardial fibrosis; cerebral stirred pulse atherosclerosis causes brain atrophy; renal stirred pulse atherosclerosis leads to hypertension or renal atrophy; and lower limb stirred pulse atherosclerosis results in intermittent claudication or gangrene.
3. Destruction of the elastic and muscular layers of the stirred pulse wall makes the wall fragile and prone to rupture and hemorrhage under blood pressure fluctuations. Cerebral stirred pulse rupture causing cerebrovascular accidents and stirred pulse aneurysm rupture leading to death are common.
The pathological progression of this disease is slow, with significant lesions often appearing after middle age, but noticeable symptoms typically emerge in old age. According to pathological data, the same degree of aortic atherosclerosis in Chinese people develops 10–15 years later on average compared to Westerners, and coronary stirred pulse atherosclerosis develops about 15–20 years later.
Numerous studies have shown that experimental stirred pulse atherosclerosis lesions, whether in early or advanced stages, can regress during periods of drug treatment and cessation of atherosclerosis-inducing diets.
bubble_chart Clinical Manifestations
The progression of this disease can be divided into four stages:
1. Asymptomatic or latent stage: The duration varies, ranging from the early pathological changes to the formation of atherosclerosis, but without clinical manifestations of organ or tissue involvement.
2. Ischemic stage: Symptoms arise due to vascular narrowing and organ ischemia.
3. Necrotic stage: Symptoms of organ or tissue necrosis occur due to intravascular thrombosis or lumen occlusion.
4. Sclerotic stage: Long-term ischemia leads to organ or tissue sclerosis (fibrosis) and atrophy, causing symptoms.
Many patients skip the necrotic stage and progress directly to the sclerotic stage, while those in the sclerotic stage may also exhibit symptoms of the ischemic stage again.
Depending on the affected artery, the disease can be classified into the following categories: ① Atherosclerosis of the aorta and its major branches; ② Coronary artery atherosclerosis; ③ Cerebral artery atherosclerosis; ④ Renal artery atherosclerosis; ⑤ Mesenteric artery atherosclerosis; ⑥ Peripheral artery atherosclerosis, etc.
Clinical manifestations primarily involve symptoms related to the affected organs.
(1) General manifestations: Decline in mental and physical abilities. Palpation of superficial arteries such as the temporal, radial, and brachial arteries may reveal widening, elongation, tortuosity, and hardening.
(2) Aortic atherosclerosis: Most cases lack specific symptoms. Percussion may reveal an expanded area of dullness behind the sternum. The second heart sound in the aortic valve area may be accentuated with a metallic tone, accompanied by a systolic murmur. Systolic blood pressure rises, pulse pressure widens, and radial artery palpation may resemble an irregular-rapid pulse. X-ray examination may show the aortic knob protruding upward and to the left, with aortic dilation and tortuosity, sometimes revealing patchy or arc-shaped calcification shadows. Aortic atherosclerosis can also lead to aortic aneurysms, most commonly occurring in the abdominal aorta below the renal artery orifice, followed by the aortic arch and descending aorta. Abdominal aortic aneurysms are often discovered during physical examinations as pulsatile abdominal masses, with murmurs audible over the corresponding abdominal wall and weakened femoral artery pulses. Thoracic aortic aneurysms may cause chest pain, dyspnea, dysphagia, hemoptysis, vocal cord paralysis due to recurrent laryngeal nerve compression, tracheal displacement or obstruction, and compression of the superior vena cava and pulmonary arteries. X-ray examination may show enlargement of the corresponding aortic segment; angiography can reveal fusiform or sac-like aneurysms. Two-dimensional ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI) can display aneurysmal aortic dilation. Aortic aneurysm rupture can be rapidly fatal. Atherosclerosis may also lead to dissecting aneurysms, though this is rare.
(3) Coronary artery atherosclerosis: Can cause angina pectoris, myocardial infarction, and myocardial fibrosis, which will be detailed in the next section.
(4) Cerebral artery atherosclerosis: Cerebral ischemia can cause vertigo, headache, and syncope. Cerebral artery thrombosis or rupture leading to hemorrhage results in cerebrovascular accidents, with symptoms such as headache, vertigo, vomiting, sudden loss of consciousness, limb paralysis, hemianopia, or aphasia (see "Acute Cerebrovascular Disease"). Brain atrophy may lead to dementia, characterized by mental abnormalities, behavioral changes, and decline in intelligence and memory, even complete personality changes (see "Mental Disorders Associated with Cerebral Artery Sclerosis").
(5) Renal artery atherosclerosis: Clinically uncommon, it can cause refractory hypertension. Sudden onset of hypertension in individuals over 55 should raise suspicion of this condition. Renal artery thrombosis may cause renal pain, anuria, and fever.
(6) Mesenteric artery atherosclerosis: May lead to symptoms such as indigestion, reduced intestinal tone, constipation, and abdominal pain. Thrombosis can cause severe abdominal pain, abdominal distension, and fever. Intestinal wall necrosis may result in hematochezia, paralytic ileus, and shock.
(7) Limbs stirred pulse Atherosclerosis is more common in the lower limbs, especially the legs. Due to impaired blood supply, it can cause coldness, numbness, and intermittent claudication in the lower limbs. This manifests as numbness, pain, or even cramps in the calf muscles during walking, which disappear after rest but reappear when walking resumes. In severe cases, persistent pain may occur, and the stirred pulse in the lower limbs—particularly the dorsal pedis stirred pulse—may weaken or disappear. If the stirred pulse lumen becomes completely blocked, gangrene may develop (see "Occlusive stirred pulse Sclerosis").
bubble_chart Auxiliary Examination
This disease still lacks sensitive and specific early laboratory diagnostic methods. Most patients exhibit abnormalities in lipid metabolism, primarily manifested as increased total cholesterol, elevated LDL cholesterol, decreased HDL cholesterol, elevated blood triglycerides, increased β-lipoprotein, elevated apolipoprotein B, decreased apolipoprotein A, increased lipoprotein (a), and abnormal lipoprotein electrophoresis patterns. Over 90% of patients present with type II or IV hyperlipoproteinemia. Hemorheological tests often show increased blood viscosity. Platelet activity may be elevated. In addition to the aforementioned manifestations of atherosclerosis, selective or computerized digital subtraction angiography can reveal luminal narrowing or aneurysmal changes caused by atherosclerosis in the coronary, cerebral, renal, mesenteric, and peripheral arteries, as well as the location, extent, and severity of the lesions. This aids in determining surgical indications and selecting the appropriate surgical approach. Doppler ultrasound is useful for assessing blood flow in peripheral and renal arteries. Limb impedance plethysmography, cerebral impedance plethysmography, electroencephalography, cerebral X-rays, computerized X-ray tomography, or magnetic resonance imaging help evaluate the functional status of peripheral and cerebral arteries and detect brain tissue abnormalities. Radionuclide studies provide insights into blood supply to the brain, heart, and kidney tissues. Echocardiography, electrocardiography, and stress tests with characteristic findings assist in diagnosing coronary atherosclerosis. Intravascular ultrasound and angioscopy offer direct visualization of atherosclerotic lesions from within the arterial lumen.
When the disease progresses to a considerable extent, especially with obvious organ lesions, diagnosis is not difficult, but early diagnosis is very challenging. In elderly patients, findings such as elevated blood lipids, narrowed blood vessels detected by stirred pulse imaging, and sexually transmitted disease changes can aid in diagnosing the condition.
bubble_chart Treatment Measures
First, active prevention of atherosclerosis should be implemented (primary prevention). If it has already occurred, aggressive treatment should be administered to halt disease progression and promote regression (secondary prevention). For patients who have developed complications, timely intervention is crucial to prevent deterioration and prolong life expectancy (tertiary prevention).
(I) General Preventive Measures
1. Encourage patients to take an active role in their treatment. Objective evidence shows that this condition can be controlled through prevention and treatment, with lesions potentially regressing partially. Patients can maintain a certain level of daily life and work capacity, and the disease itself may promote the formation of collateral circulation in the arteries, improving the condition. Therefore, persuading patients to patiently adhere to long-term preventive measures is essential.
2. Balanced Diet
(1) Total caloric intake should not be excessive, aiming to maintain a normal weight, especially for those over 40 to prevent obesity. A simple formula for calculating normal weight is: height (in cm) minus 110 equals weight (in kg), which can serve as a reference.
(2) For those exceeding the standard weight, daily caloric intake should be reduced. A low-fat diet (fat intake not exceeding 30% of total calories, with animal fats limited to 10%) and low-cholesterol diet (no more than 500mg daily) are recommended, along with restricted consumption of sucrose and sugary foods.
(3) Individuals over 40, even without elevated blood lipids, should avoid frequent consumption of excessive animal fats and vegetable oils high in saturated fatty acids, such as fatty meat, lard, bone marrow, cream and its products, coconut oil, and cocoa oil. They should also limit foods high in cholesterol, such as organ meats (liver, brain, kidney, lung), squid, oyster shells, cuttlefish, fish roe, shrimp roe, crab roe, and egg yolks. If blood lipids remain elevated, a diet low in cholesterol and animal fats—such as lean meats, poultry, fish, egg whites, and soy products—should be adopted.
(4) Patients diagnosed with coronary atherosclerosis must avoid overeating to prevent triggering angina or myocardial infarction. Those with hypertension or heart failure should also limit salt and sodium-rich foods.
(5) A light diet is encouraged, with emphasis on foods rich in vitamin C (e.g., fresh vegetables and fruits) and plant proteins (e.g., legumes and their products). Whenever possible, use oils such as soybean oil, rapeseed oil, sesame oil, corn oil, tea-seed oil, or rice bran oil for cooking.
3. Appropriate Physical Activity and Exercise Engaging in physical labor and sports activities helps prevent obesity, enhances circulatory function, and regulates lipid metabolism, making it a proactive measure against this disease. Physical activity should be tailored to the individual’s baseline health, activity habits, and cardiac function, avoiding excessive strain or discomfort. Exercise should be gradual; strenuous activities are not recommended. For the elderly, walking (one hour daily, divided into sessions), health exercises, and tai chi are encouraged.
4. Balanced Work and Lifestyle Maintain a regular routine, stay optimistic and cheerful, avoid overexertion and emotional stress, balance work and rest, and ensure adequate sleep.
5. Avoid Smoking and Excessive Alcohol Refrain from smoking and heavy alcohol consumption (moderate intake of low-alcohol beverages may increase HDL levels).
6. Treat Comorbid Conditions Actively manage diseases related to atherosclerosis, such as hypertension, obesity, hyperlipidemia, gout, diabetes, liver disease, nephrotic syndrome, and relevant endocrine disorders.
Some suggest that preventive measures should begin in childhood, avoiding high-cholesterol, high-fat diets and overeating to prevent obesity.
(II) Pharmacological Treatment
1. Vasodilators These drugs alleviate vascular motility disorders (refer to "angina" and "obliterative atherosclerosis").
2. Lipid-lowering drugs For patients with elevated blood lipids who still have levels above normal after dietary adjustments and physical activity (total cholesterol >5.2mmol/L [200mg/dL], LDL cholesterol >3.4mmol/L [130mg/dL], triglycerides >1.24mmol/L [110mg/dL]), the following lipid-lowering medications may be selected based on individual circumstances:
⑴Drugs that only lower blood cholesterol
1) Bile acid sequestering resins: These are anion exchange resins. After ingestion, they adsorb bile acids in the intestines, block the enterohepatic circulation of bile acids, accelerate the breakdown of cholesterol into bile acids in the liver, and excrete them along with intestinal bile acids, thereby reducing blood cholesterol. Cholestyramine can be used 3 times/day, 4-5g each time; Colestipol 3-4 times/day, 4-5g each time; Sephadex (DEAE) 3-4 times/day, 4g each time. Gastrointestinal reactions such as constipation are prone to occur, and they may affect the absorption of fat-soluble vitamins, making them less tolerable for patients. New micro-particle formulations act faster with fewer side effects.
2) Probucol: It interferes with the cholesterol acetate biosynthesis stage in the liver, lowering blood cholesterol and LDL, but also reduces HDL. 500mg twice daily. Side effects include gastrointestinal reactions, headache, vertigo, etc.
3) Neomycin: Oral administration increases bile salt excretion in feces, reduces cholesterol absorption, and lowers blood cholesterol and LDL. 2g/day taken at bedtime. Side effects include nausea, abdominal pain, diarrhea, and potential hearing and kidney function impairment.
⑵Drugs that primarily lower blood cholesterol but also reduce blood triglycerides
1) Statins: These are HMG-CoA reductase inhibitors that inhibit cholesterol synthesis, accelerate LDL clearance, and reduce blood cholesterol and LDL levels. They can also lower blood triglycerides and VLDL while increasing HDL and apolipoprotein AII levels. They represent a new class of drugs. Options include Lovastatin 20mg 1-2 times/day, Pravastatin 20mg 1-2 times/day, Simvastatin 10-40mg 1-2 times/day, and Fluvastatin 20-40mg once daily. Side effects include myalgia, gastrointestinal symptoms, insomnia, rash, and elevated transaminases.
2) Elastase: This is a soluble elastin that inhibits cholesterol synthesis and promotes cholesterol conversion into bile acids, thereby lowering blood cholesterol. 300u three times daily. It has weaker effects but fewer side effects.
⑶Drugs that primarily lower blood triglycerides but also reduce blood cholesterol.
1) Fibrates: The earliest used was clofibrate, 3-4 times a day, 0.5g each time. Its effect on lowering blood triglycerides is stronger than that on cholesterol, and it also increases HDL. Additionally, it reduces cholesterol deposition in tissues, decreases platelet adhesion, enhances fibrinolytic activity, and lowers fibrinogen concentration, thereby inhibiting blood coagulation. When used with anticoagulants, the dose of the anticoagulant should be readjusted. A few patients may experience gastrointestinal reactions, skin itching, urticaria, transient increases in serum transaminase, and changes in kidney function. Regular liver and kidney function tests are advisable. Long-term use increases the incidence of gallstones. It has now been replaced by newer drugs of the same class, such as fenofibrate (3 times a day, 100mg each time), etofylline clofibrate (2-3 times a day, 250mg each time), gemfibrozil (2 times a day, 600mg each time), bezafibrate (3 times a day, 200mg each time), and ciprofibrate (once a day, 50-100mg each time).
2) Nicotinic acid (nicotinic acid) class: Inhibits liver synthesis of VLDL, thereby reducing blood triglycerides, cholesterol, and LDL while increasing HDL. It also dilates peripheral blood vessels. Taken after meals, the dose starts at 0.1g and gradually increases to a maximum of 1.0g. Side effects include skin flushing, itching, gastric discomfort, etc. Long-term use requires monitoring of liver function. Nicotinic acid derivatives with fewer side effects commonly used include inositol hexanicotinate, taken 3 times/day, 0.4–0.6g each time; dl-α-tocopherol nicotinate, taken 3 times/day, 100–300mg each time; and acipimox, taken 3 times/day, 0.25g each time.
3) Unsaturated fatty acids (unsaturated fatty acid): Fish oil contains large amounts of n-3 unsaturated fatty acids, such as docosahexaenoic acid (DHA). Vegetable oils contain more n-6 unsaturated fatty acids, such as linoleic acid. They can inhibit lipid absorption in the small intestine and the reabsorption of bile acids, possibly suppressing liver lipid and lipoprotein synthesis while promoting cholesterol excretion in feces. They help lower blood triglycerides, VLDL, cholesterol, and LDL while increasing HDL. Additionally, they inhibit platelet function and reduce thrombus formation. Fish oil preparations can be taken twice daily, 5–10g each time; Duoxikang pills, 3 times/day, 1.8g each time; linoleic acid pills, 3 times/day, 300mg each time. Others include evening primrose oil and rubber seed oil. However, unsaturated fatty acids are highly prone to oxidation, forming substances that promote atherosclerosis, and may cause gastrointestinal reactions, so they should be used cautiously.
4) Pantethine: A component of coenzyme A, it promotes normal lipid metabolism, reducing blood triglycerides and cholesterol while increasing HDL. Taken 3 times/day, 200mg each time. Side effects are minimal, but its effects are relatively weak.
4) Other drugs: Dextran sulfate, β-sitosterol, sodium alginate diester, vitamin C, vitamin B6, etc., have also been used as lipid-regulating drugs.
5) Chinese herbal medicine: Alisma, Polygonum multiflorum, barley root, tea tree root, water-ground thistle, hawthorn fruit, germinated barley, Chinese Taxillus herb, giant knotweed, Panax notoginseng, Pueraria root, Solomonseal rhizome, Cassia seed, Ganoderma, Polyghace seche, Typha, garlic, Chinese caterpillar fungus, Gynostemma pentaphyllum, etc., have all been reported to have lipid-lowering effects.
Lipid-regulating drugs often require long-term use, so careful attention should be paid to dosage and side effects. Due to their adverse side effects, previously used lipid-lowering drugs like estrogen and dextrothyroxine have been phased out.
3. Antiplatelet drugs Antiplatelet aggregation and adhesion drugs can prevent thrombosis and may help prevent vascular occlusion, sexually transmitted disease changes, and disease progression. They can be used to prevent recurrence after myocardial infarction and to prevent cerebral stirred pulse thromboembolism: ① Aspirin 0.3g/d or a smaller dose of 50mg/d works by inhibiting the production of TXA2 while having less effect on PGI2 production. ② Dipyridamole (Persantin) 50mg three times a day can increase cyclic adenosine monophosphate in platelets and prolong platelet lifespan. It can be used at half the dose in combination with aspirin. ③ Sulfinpyrazone 0.2g three times a day has effects similar to aspirin, and some reports suggest it may prevent sudden death from coronary stirred pulse atherosclerotic heart disease. ④ Ticlopidine 250mg twice a day has the same effect as dipyridamole and also has a membrane-stabilizing effect on platelets similar to clofibrate. ⑤ Fenflumizole, an imidazole derivative and TXA2 synthase inhibitor, is taken at 50mg twice a day.
4. There are also some proteoglycan preparations such as chondroitin sulfate A and C (1.5g three times a day) and coronary heart soothing agent (20mg three times a day), which exert therapeutic effects by adjusting the proteoglycan structure of the arterial wall.
(3) Surgical Treatment
This includes surgical procedures such as recanalization, reconstruction, or bypass grafting for stenotic or occluded blood vessels, especially coronary arteries, aorta, renal arteries, and peripheral arteries. Interventional treatments such as transluminal angioplasty using balloon catheters, transluminal laser recanalization, transluminal rotational atherectomy or rotational grinding of atherosclerotic plaques, and stent placement after transluminal angioplasty can also be performed. In addition, for hypercholesterolemia that is unresponsive to drug therapy, ileal bypass surgery or plasma exchange therapy has been performed abroad, but these methods are expensive or may have sequelae.
The prognosis of this disease varies depending on the location and severity of the lesions, the rate of vascular stenosis progression, the extent of organ involvement, and the presence of complications. Patients with brain, heart, or kidney involvement leading to cerebrovascular accidents, myocardial infarction, or renal failure have a poor prognosis.
The changes and aneurysms of the aorta caused by atherosclerosis must be differentiated from syphilitic aortitis, aortic aneurysms, and mediastinal tumors; the angina pectoris and myocardial infarction caused by coronary atherosclerosis must be distinguished from those caused by other coronary artery diseases; myocardial fibrosis must be differentiated from other heart diseases, especially cardiomyopathy; the cerebrovascular accidents caused by cerebral atherosclerosis must be distinguished from those caused by other reasons; the hypertension caused by renal atherosclerosis must be differentiated from hypertension due to other causes; renal artery thrombosis must be distinguished from renal calculi; the symptoms caused by atherosclerosis of the extremities must be differentiated from those caused by other arterial diseases.






