| disease | Pulmonary Stenosis |
Pulmonary stenosis refers to the narrowing of the right ventricular outflow tract, pulmonary valve, or pulmonary trunk and its branches. It can occur alone or as part of other cardiac malformations, such as tetralogy of Fallot. Its incidence accounts for about 10% of congenital heart diseases. Among pulmonary stenosis cases, pulmonary valve stenosis is the most common, accounting for about 90%, followed by infundibular stenosis, while stenosis of the pulmonary trunk and its branches is rare. The embryonic developmental causes of various types of pulmonary stenosis differ. During the sixth week of embryonic development, the truncus arteriosus begins to divide into the aorta and pulmonary artery. Within the pulmonary artery cavity, membranes start forming three primitive valve nodules, which grow inward and later thin out to form the three pulmonary valve leaflets. If the development of these membranes is disrupted, the three valve leaflets may fuse into a dome-shaped, protruding orifice, resulting in pulmonary valve stenosis. Simultaneously, during the development of the pulmonary valve, the conus arteriosus of the bulbus cordis is absorbed to form the right ventricular outflow tract (i.e., the infundibulum). If this process is impaired, circular muscle hypertrophy or hypertrophic muscle bundles spanning the ventricular wall and septum may form, leading to infundibular stenosis. Additionally, during embryonic development, the sixth aortic arch develops into the left and right pulmonary arteries, with their distal ends connecting to the small pulmonary arteries and their proximal ends linking to the pulmonary trunk. If this development is disrupted, stenosis of the pulmonary artery branches or pulmonary trunk may occur.
bubble_chart Pathological ChangesPathological anatomy: Pulmonary valve stenosis: The three valve leaflets are fused into a dome-shaped thickened membrane, protruding into the lumen. The valve orifice appears fish-mouth-like, either centrally or eccentrically located. The smaller orifice measures only 2–3 mm, while the general orifice ranges from 5 to 12 mm. At the fused commissures of the valve leaflets, a slightly raised ridge is often present. In most cases, all three leaflets are fused, while in a minority, only two leaflets are fused. The valve edges are often thickened, with verrucous nodules, and occasionally calcified ecchymoses may form. The pulmonary valve annulus generally exhibits varying degrees of stenosis. Due to blood flow obstruction, the right ventricle hypertrophies, which can lead to secondary hypertrophic stenosis of the right ventricular outflow tract and right ventricular dilation, resulting in tricuspid valve insufficiency.
The pulmonary trunk may exhibit post-stenotic fusiform dilation, often extending to the left pulmonary artery. The pulmonary trunk is significantly larger than the main artery.
Infundibular stenosis presents in two types: The first is the membranous type of stenosis, where below the conus, a fibromuscular membrane forms between the supraventricular crest and the parietal band of the right ventricular outflow tract, dividing the right ventricle into two chambers of unequal size. The upper chamber, with thin walls and slight dilation, is called the third ventricle, while the lower chamber is the hypertrophied right ventricle. A narrow orifice, ranging from 3 to 15 mm, exists in the center of the separating membrane. This membranous type of stenosis often coexists with pulmonary valve stenosis, termed mixed stenosis. The second type is the tubular stenosis, primarily characterized by diffuse muscular hypertrophy of the right ventricular outflow tract wall, forming a long, narrow channel. This type is often accompanied by hypoplasia of the pulmonary valve annulus and pulmonary trunk, thus lacking post-stenotic dilation of the pulmonary artery.
Pathophysiology: Regardless of the type of pulmonary stenosis, it obstructs right ventricular outflow, increasing the pressure within the right ventricular cavity. The degree of pressure elevation is proportional to the severity of the pulmonary stenosis. Pulmonary artery pressure remains normal or slightly decreased, creating a transvalvular pressure gradient between the right ventricle and the pulmonary artery. This gradient increases with the severity of the stenosis. If the transvalvular pressure gradient is below 5.3 kPa (40 mmHg), it is classified as grade I pulmonary stenosis, which minimally affects right ventricular outflow. When the gradient ranges between 5.34 and 13.33 kPa (40–100 mmHg), it is considered moderate pulmonary stenosis, and right ventricular outflow begins to be impaired, especially during exercise, reducing cardiac output. If the gradient exceeds 13.33 kPa (100 mmHg), right ventricular outflow is significantly obstructed, even at rest, leading to reduced right ventricular output and markedly increased right ventricular load. Over time, this promotes right ventricular hypertrophy, myocardial strain, and dilation of the right ventricular cavity, causing tricuspid annular dilation and relative tricuspid insufficiency. Subsequently, right atrial pressure increases, leading to right atrial hypertrophy. When right atrial pressure exceeds left atrial pressure, and if the foramen ovale is patent, blood may shunt from the right atrium to the left atrium, resulting in central cyanosis clinically. Prolonged right ventricular load may ultimately lead to right heart failure, manifesting as jugular vein distension, hepatomegaly, ascites, and lower limb edema. bubble_chart Clinical ManifestationsThe male-to-female ratio of this disease is approximately 3:2, with the onset age mostly between 10 and 20 years. The symptoms are closely related to pulmonary {|###|} stenosis. Patients with grade I pulmonary {|###|} stenosis are generally asymptomatic, but symptoms gradually appear with age, mainly manifested as poor exercise tolerance, {|###|} lack of strength, and palpitation, shortness of breath after exertion. Patients with grade III stenosis may experience dizziness or {|###|} syncope episodes. In advanced stage cases, symptoms of right {|###|} heart failure such as jugular vein distension, hepatomegaly, and lower limb edema may occur. If accompanied by atrial septal defect or patent foramen ovale, cyanosis of the lips or peripheral fingers (toes) and clubbing of fingers (toes) may be observed.
Physical examination Most patients are well-developed. The main {|###|} sign is a loud, rough, ejection-type systolic murmur of grade III–IV heard at the second left intercostal space near the sternum, radiating to the left neck or left subclavian region. A systolic {|###|} tremor can be palpated at the site where the murmur is loudest. The intensity of the murmur varies depending on the degree of stenosis, blood flow velocity, blood flow volume, and chest wall thickness. The second heart sound in the pulmonary {|###|} valve area is often weakened or split. In patients with {|###|} fistula disease and infundibular stenosis, the murmur and {|###|} tremor are usually located at the left third or fourth intercostal space, with milder intensity. The second heart sound in the pulmonary {|###|} valve area may not be weakened and may even appear split.
Patients with grade III pulmonary {|###|} orifice stenosis may exhibit left sternal border protrusion due to right ventricular hypertrophy, and a lifting impulse can be palpated in the precordial area. Due to relative tricuspid insufficiency, a blowing systolic murmur can be heard in the tricuspid area. When right-to-left shunting of blood occurs in the atrium, the patient may develop cyanosis of the lips and clubbing of the fingers (toes).
bubble_chart Auxiliary Examination
X-ray examination: Grade I pulmonary artery stenosis may show no abnormalities on chest X-ray, while moderate and Grade III stenosis cases demonstrate Grade I or Grade II enlargement of the cardiac shadow, primarily characterized by hypertrophy of the right ventricle and right atrium. The apex of the heart appears spherical and elevated due to right ventricular hypertrophy. In cases of pulmonary valve stenosis, the dilated pulmonary artery segment bulges outward in a rounded shape, whereas in patients with infundibular stenosis, this segment appears flat or even depressed. The hilar vascular shadows are reduced, and the pulmonary vascular markings are fine, especially in the outer third of the lung fields, resulting in clear lung fields (Figure 2).
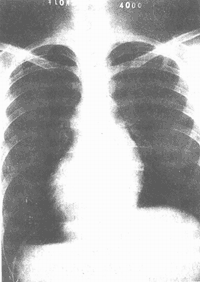
Figure 2: X-ray findings of pulmonary valve stenosis. The cardiac shadow shows mild to Grade II enlargement, with a rounded and elevated apex, outward bulging of the pulmonary artery segment, and clear lung fields.
Electrocardiogram (ECG) examination: ECG changes vary depending on the severity of stenosis. Patients with Grade I pulmonary artery stenosis may have normal ECG findings, while those with Grade II or higher stenosis exhibit right axis deviation, right ventricular hypertrophy, strain, and inverted T waves. Grade III stenosis cases may display tall and peaked P waves indicative of atrial hypertrophy. Some cases may also show incomplete right bundle branch block.
Echocardiography: In cases of pulmonary valve stenosis, echocardiography can reveal restricted leaflet opening, dome-shaped protrusion of the valve leaflets, and a narrowed valve orifice. It can also assess the degree of right ventricular outflow tract muscle hypertrophy and the enlargement of the right ventricle and right atrium.
Based on clinical signs, X-ray, and echocardiography, a preliminary diagnosis of pulmonary artery stenosis can generally be made. However, for certain cases, further diagnostic clarification or differentiation may be necessary to determine the severity of stenosis and associated cardiac anomalies, which aids in selecting the appropriate surgical approach. Right heart catheterization or right ventricular angiography may be required.
Right heart catheterization and selective right ventricular angiography: The normal right ventricular systolic pressure is 2.0–4.0 kPa (15–30 mmHg), and the diastolic pressure is 0–0.7 kPa (0–5 mmHg). The pulmonary artery systolic pressure is consistent with the right ventricular systolic pressure. If the right ventricular systolic pressure exceeds 4.0 kPa (30 mmHg) and the pressure gradient between the right ventricle and pulmonary artery exceeds 1.3 kPa (10 mmHg), pulmonary artery stenosis is likely present. The magnitude of the transvalvular pressure gradient reflects the severity of stenosis: a gradient below 5.3 kPa (40 mmHg) indicates Grade I stenosis, with a valve orifice of approximately 1.5–2.0 cm; a gradient of 5.3–13.3 kPa (40–100 mmHg) indicates Grade II stenosis, with a valve orifice of about 1.0–1.5 cm; and a gradient above 13.3 kPa (100 mmHg) indicates Grade III stenosis, with an estimated valve orifice of 0.5–1.0 cm. Continuous pressure recording during catheter withdrawal from the pulmonary artery to the right ventricle, along with analysis of pressure curve patterns and the presence of a third type of curve, can determine whether the stenosis is purely valvular, infundibular, or a mixed type (Figure 3).
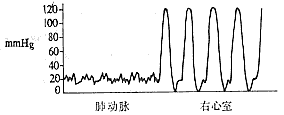
① Valvular type
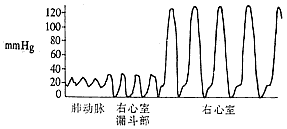
② Infundibular type
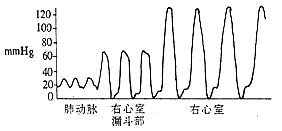
③ Mixed type
Figure 3: Continuous pressure recording between the pulmonary artery and right ventricle, indicating the site of pulmonary artery stenosis.
Note: 1 mmHg = 0.133 kPa.
Selective right ventricular angiography is not necessary as a routine examination, but for some difficult cases where a clear diagnosis and differential diagnosis are required to understand the location and degree of stenosis, it can be combined with right heart catheterization to perform right ventricular angiography. Contrast medium is injected into the right ventricle, and the outflow of contrast is obstructed at the pulmonary valve area. The valve membrane is fused and protrudes dome-shaped into the pulmonary artery cavity. The contrast medium is ejected through the narrow valve orifice into the pulmonary artery and then spreads out in a fan shape. In cases of infundibular stenosis, a narrow and elongated contrast image can be observed in the right ventricular outflow tract.
bubble_chart Treatment Measures
Grade I pulmonary stenosis patients are clinically asymptomatic, can grow and develop normally, and adapt to normal living capacity without requiring surgical treatment. Moderate pulmonary stenosis patients generally develop palpitations and shortness of breath after activity around the age of 20. Without surgical intervention, the increasing age will inevitably lead to excessive right ventricular load, resulting in right heart failure symptoms, thereby losing the ability to live and work. For severe Grade III pulmonary stenosis patients, obvious symptoms often appear in early childhood, and without timely treatment, death may occur during infancy.
(1) Surgical Indications: ① Patients without symptoms and no significant abnormalities on electrocardiogram, but with right ventricular systolic pressure above 8.0 kPa (60 mmHg) on right heart catheterization, or a transvalvular pressure gradient greater than 5.3 kPa (40 mmHg), or echocardiography showing a valve orifice of 1.0–1.5 cm (Grade II stenosis), should be considered for surgery. ② Asymptomatic patients with electrocardiographic evidence of right ventricular hypertrophy or strain, or chest X-ray showing Grade II cardiac enlargement. ③ Symptomatic patients with abnormal electrocardiographic and X-ray findings; the optimal surgical age is preschool. ④ Patients with obvious symptoms and a history of syncope, indicating severe stenosis, should undergo surgery during infancy to reduce right ventricular load.
(2) Surgical Methods: Pulmonary valve stenosis was traditionally treated under moderate hypothermia (30–32°C) with direct vision commissurotomy. Hypothermia is simple, causes minimal disruption to physiological functions, and ensures smooth postoperative recovery. However, hypothermia only allows a safe circulatory arrest time of 6–8 minutes, requiring rushed intracardiac procedures without sufficient time to explore and correct intracardiac anomalies. In recent years, advancements in extracorporeal circulation, myocardial protection, and surgical techniques have made open-heart surgery safer. Thus, pulmonary valve stenosis surgery is now generally performed under extracorporeal circulation with direct vision correction.
1. Moderate Hypothermia Pulmonary Valve Commissurotomy: Suitable only for simple pulmonary valve stenosis with mild conditions and no secondary infundibular stenosis or associated intracardiac anomalies.
After tracheal intubation anesthesia, the patient is immersed in ice water for surface cooling. When nasopharyngeal temperature drops to 35°C, the patient is removed, dried, and body temperature continues to decline to 32–30°C.
The patient is placed in a supine position, and a median sternotomy is performed. After pericardiotomy, external palpation reveals a systolic thrill at the pulmonary artery root, with thickened, hardened, and fused valve leaflets. The right ventricle shows significant hypertrophy and enlargement. After examination, the superior and inferior vena cavae are isolated and encircled with tourniquets. Four traction sutures are placed on the anterior wall of the pulmonary artery. The anterior wall is partially clamped and incised longitudinally. After hyperventilation by the anesthesiologist, the vena cavae are occluded, and caval blood flow is blocked. After 6–8 heartbeats to empty the cardiac chambers, the pulmonary artery clamp is released, and blood is aspirated. Retractors expose the pulmonary valve and annulus. The fused commissures are identified and incised with scissors or a scalpel from the valve orifice along the fusion ridge toward the annulus. A finger is inserted through the incised valve to assess right ventricular hypertrophy and stenosis. After completing the intracardiac procedure, the pulmonary artery traction sutures are lifted, the superior vena cava tourniquet is released, and assisted ventilation resumes. Blood gushes from the pulmonary artery incision, and residual air is expelled before clamping the incision. The incision is sutured with fine thread, and the inferior vena cava tourniquet is released after 30 seconds. Transient reactive hypertension may occur postoperatively. After blood pressure and heart rate normalize, the pericardium is sutured, and drainage tubes are placed in the pericardium and retrosternally. If ventricular fibrillation occurs during surgery, the stenotic valve should be rapidly incised, followed by cardiac massage and defibrillation (Figure 1).
⑴ Four traction lines and incision lines of the lung's stirred pulse
⑵ After blocking the superior and inferior vena cava, the narrowed pulmonary valve is incised along the commissural ridge through the pulmonary incision.
Lower right figure: Incising the fused valve along the commissure.
⑶ Finger retrograde exploration of the right ventricular infundibulum.
⑷ Open the superior vena cava, lift the four traction sutures, and perform pulmonary valve exhaust.
⑸ After clamping the pulmonary incision, suture it closed.
Figure 1: Pulmonary valve stenosis under hypothermic direct vision valve incision.
2. Direct vision correction under extracorporeal circulation: Suitable for the treatment of various types of pulmonary stenosis. The patient's position, chest incision, and anatomical steps of the heart and great vessels are the same as in hypothermic direct vision surgery. After heparinization at 3mg/kg, an arterial perfusion cannula is inserted into the ascending aorta, and superior and inferior vena cava drainage cannulas are inserted via the right atrial appendage and right atrium, respectively. After establishing extracorporeal circulation, blood cooling begins. Depending on cardiac function and the complexity of the lesion, the patient's nasopharyngeal temperature is lowered to between 32-28°C. After aortic cross-clamping, cold cardioplegic solution is rapidly injected into the root of the ascending aorta, and the pericardial cavity is irrigated with ice water. The heart surface is locally cooled with small ice bags to protect the myocardium. Then, the superior and inferior vena cava are blocked with tapes, and the pulmonary artery or right ventricle is incised to perform direct vision commissurotomy of the pulmonary valve and/or infundibular muscle resection with outflow tract patch enlargement.
Infundibular muscle resection should include the septal band, parietal band, and part of the hypertrophied supraventricular crest. Intracardiac hypertrophic trabeculae should be excised to clear the outflow tract, but care must be taken to avoid cutting adjacent papillary muscles, conus muscles, and moderator bands. The diameter of the cleared outflow tract should be greater than 1.7 cm in adults and about 1.4 cm in children; otherwise, a pericardial patch should be tailored into a spindle shape to enlarge the outflow tract. A narrow pulmonary valve annulus should also be incised until the right ventricular outflow tract is reached, then enlarged with a fabric or pericardial patch. A transannular patch may cause pulmonary valve insufficiency, potentially leading to postoperative right ventricular failure. To avoid severe pulmonary valve insufficiency and safely pass the postoperative critical period, a patch with valve leaflets can be used to enlarge the pulmonary valve annulus, preventing insufficiency due to annular enlargement. After the procedure, needles are inserted into the right ventricle and aortic root for air removal, and the aortic cross-clamp is released. If necessary, electrical defibrillation is used to restore heart rhythm. After rewarming to 35°C, with strong heartbeats and stable blood pressure, extracorporeal circulation is stopped. The superior and inferior vena cava cannulas and arterial perfusion cannula are removed sequentially. Protamine is administered intravenously at 4.5 cm per kilogram of body weight to neutralize heparin.
Postoperative complications and surgical outcomes: In addition to the general complications of extracorporeal circulation open-heart surgery, there are two main postoperative complications: First, after relieving pulmonary stenosis, pulmonary blood volume significantly increases. Therefore, blood volume should be appropriately replenished based on arterial pressure and central venous pressure to avoid postoperative low cardiac output syndrome. If necessary, intravenous inotropes like dopamine and cedilanid can be administered to enhance myocardial contractility until hemodynamic stability is achieved. Second, if outflow tract stenosis is not fully relieved and right ventricular pressure remains high, postoperative right ventricular incision bleeding is likely, and right heart failure may easily occur.
The surgical mortality rate for this condition is relatively low, generally around 2%. Surgical outcomes are satisfactory, with postoperative symptoms improving or completely disappearing, allowing patients to resume normal life.




