| disease | Spinal Tuberculosis Complicated by Paraplegia |
Spinal subcutaneous node complicated with paraplegia is caused by a combination of factors such as cavity fluid, caseous material, dead bone, or necrotic intervertebral disc in the lesion. In the advanced stage of the disease, the spinal cord may be surrounded by fibrous scar tissue of granulation within the spinal canal, along with pathological dislocation or subluxation of the vertebral body. According to Sorrel and Sorrel-Dejerin (1925), paraplegia occurring within 2 years of the course of spinal subcutaneous node is referred to as early-onset paraplegia, while paraplegia occurring after 2 years is termed delayed-onset paraplegia.
bubble_chart Etiology
I. Key Points of Anatomy
1. Composition of the Vertebral Canal
The vertebral canal is formed by the connection of the vertebral foramina of each vertebra. Its upper end starts from the foramen magnum of the occipital bone, and its lower end terminates at the sacral hiatus. The anterior wall is composed of the vertebral bodies, intervertebral discs, and the posterior longitudinal ligament, while the posterior wall is formed by the vertebral arches and the ligamentum flavum. The vertebral canal is widest at the lower cervical and lumbar regions, and narrower at the mid-cervical and thoracic regions. Therefore, the aforementioned areas are relatively
2. Contents of the Vertebral Canal
The vertebral canal contains the spinal cord, spinal membranes, spinal nerve roots, venous plexuses, and adipose tissue.
(1) The spinal cord is covered by three membranes from outside to inside: the dura mater, arachnoid mater, and pia mater, which are continuous with the three meninges of the brain.
(2) Spinal Membrane Cavities① The subarachnoid space lies between the arachnoid mater and pia mater and communicates with the intracranial ventricles and the subarachnoid space of the brain. It is filled with cerebrospinal fluid. The subarachnoid space at the L2~S2 level is wider and is called the terminal cistern, where there is more cerebrospinal fluid. This cavity contains only the cauda equina and the filum terminale, and lumbar punctures and anesthesia are performed through this space.
② The epidural space is the cavity between the dura mater and the vertebral canal. It is filled with adipose tissue and venous plexuses and is under negative pressure.
(3) The internal vertebral venous plexus is located within the epidural space and is divided into anterior and posterior plexuses, located on the anterior and posterior walls of the vertebral canal, respectively. They receive blood from the vertebrae and spinal cord and drain into the vertebral veins at the intervertebral foramina. These veins drain into the vertebral veins in the neck, the azygos and hemiazygos veins in the thorax, and the lumbar veins in the lumbar region.
3. Blood Supply of the Spinal Cord The blood supply to the spinal cord mainly comes from the following sources:
(1) The anterior spinal artery arises from the vertebral artery, with the left and right branches merging into one, descending along the anterior median fissure of the spinal cord. It gives off branches that penetrate the spinal cord, reaching the anterior horn, lateral horn, central gray matter, and the deep parts of the anterior and lateral funiculi, supplying the anterior two-thirds of the spinal cord.
(2) The posterior spinal artery arises from the vertebral artery or the posterior inferior cerebellar artery, with one branch on each side descending along the posterolateral sulcus medial to the posterior roots. It anastomoses with the segmental and posterior root arteries, mainly supplying the posterior one-third of the spinal cord.
(4) The radicular arteries arise from the ascending cervical artery, intercostal arteries, and lumbar arteries, entering the vertebral canal through the intervertebral foramina and anastomosing with the anterior and posterior spinal arteries. This ensures that the anterior and posterior spinal arteries are continuously supplemented and reinforced with blood as they descend. The spinal cord receives blood from different sources, with the anterior radicular arteries supplying about 6~10 branches, located in the cervical region with 0~6 branches. In the cervical region, there are 0~6 roots, in the thoracic region 2~4 roots, and in the lumbar region 1~2 roots. One large anterior radicular artery is called the artery of Adamkiewicz. There are about 10~23 posterior radicular arteries, distributed on the dorsal side of the spinal cord and anastomosing with a pair of posterior spinal arteries. The radicular arteries are often more numerous on the left side than the right in the thoracolumbar region.
The transitional zones between different blood supply sources in various segments of the spinal cord are most susceptible to ischemic damage. For example, the upper thoracic spinal cord is mainly supplied by branches of the intercostal arteries. When several adjacent intercostal arteries are injured or ligated, the anterior spinal artery may not provide sufficient blood to that segment of the spinal cord, particularly the fourth thoracic segment, which is most vulnerable. Similarly, the first lumbar segment is also a transitional zone between the upper and lower radicular arteries and is prone to damage (Figure 1).
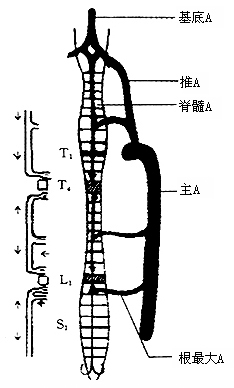
Figure 1: Schematic of the arterial blood supply to the spinal cord T4L1 is a relatively ischemic zone.
2. Causes and classification of paraplegia associated with spinal subcutaneous nodules
The classification of paraplegia aims to provide an objective basis for selecting treatment options, comparing treatment outcomes, and determining prognosis.
1. Active Lesion Type Paraplegia
The spinal cord is subjected to a pressure of 2~2.66Pa (15~20mmHg) due to cavity fluid, caseous material, and granulation tissue (soft compressive substances) in the lesion; local vascular embolism and spinal cord edema caused by dead bone or necrotic intervertebral disc (hard compressive substances); in rare cases, paraplegia is caused by comprehensive reasons such as subcutaneous nodular granulation tissue penetrating the dura mater, leading to subcutaneous nodular spinal meningitis (Hodgson et al. 1967). This type accounts for about 89% of paraplegia cases, and except for cases of vascular embolism and subcutaneous nodular spinal meningitis, the treatment effect is generally good (Figure 2).
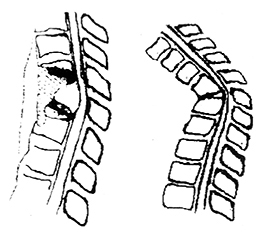
Figure 2 Classification of Spinal Subcutaneous Nodule Paraplegia
① Active Lesion Type Paraplegia ② Healed Lesion Type Paraplegia
2. Healed Lesion Type Paraplegia
In the advanced stage of the disease, in addition to the spinal cord being surrounded by fibrous scar tissue from granulation tissue within the spinal canal, pathological dislocation or semi-dislocation of the vertebral body, especially in the upper thoracic and thoracolumbar segments, can lead to spinal kyphosis, elongation of the spinal canal, excessive stretching and tension of the spinal cord over the anterior bony ridge of the spinal canal, atrophy or degeneration, wear, and other causes of paralysis. This type accounts for about 11% of paraplegia cases and has a poor prognosis.
bubble_chart Clinical Manifestations
Spinal subcutaneous node lesion activity paraplegia, usually accompanied by symptoms of systemic intoxication such as fatigue, low-grade fever in the afternoon, and night sweating, which distinguishes it from paraplegia caused by other diseases. In patients with cured bone lesions, systemic subcutaneous nodular symptoms are often not obvious.
1. Motor impairment
Usually, paraplegia appears after spinal subcutaneous nodules, with a few cases presenting paraplegia as the initial symptom. The progression of paraplegia is mostly slow, starting with spinal cord conduction tract disorders, manifesting as spontaneous muscle twitching in the lower limbs, clumsy gait, weakness, and easy falls. In cases of spinal subcutaneous nodules combined with high-level paralysis, paralysis of the upper limbs and chest-wall muscles, and after intercostal muscle paralysis, thoracic autonomous breathing weakens, relying on diaphragmatic activity to maintain gas exchange. Long-term bedridden patients, especially the elderly, are prone to complications such as atelectasis or pneumonia and should be prevented. Pathological reflexes are positive, with hyperactive tendon reflexes, patellar and Achilles tendon clonus, etc. The progression of paraplegia often shifts from spastic paresis to spastic extension-type paraplegia, then to spastic flexion-type paraplegia, indicating complete compression of the pyramidal and extrapyramidal tracts. In the most severe cases, patients rapidly transition from spastic paraplegia to flaccid paraplegia, resembling spinal shock.
2. Sensory impairment
Generally, sensory impairment of varying degrees appears after severe motor impairment in the lower limbs. Sensory is divided into superficial and deep groups, with superficial sensations including pain, temperature, and touch. Deep sensations include tremor sense, deep touch, and position sense. Mild sensory impairment may present as hypersensitivity, such as hypersensitivity to cold, heat, and pain in the affected limb, while more severe cases may show sensory dullness, and in severe cases, sensory loss. Determining the sensory level is crucial for identifying the level of spinal cord compression. Cervical 2~3~4 forms the cervical plexus, innervating the occipital, neck, anterior neck, and skin above the second rib of the anterior chest. Cervical 4~thoracic 1 lesion sensory impairment level is at the second rib. Thoracic 3~4 sensory impairment level is at the nipple level, thoracic 6 lesion level at the xiphoid process, thoracic 9 at the umbilicus, thoracic 12 lesion at the groin. Bladder and anal sphincter dysfunction, distal limb position sense, and vibration sense are the last to disappear (Figure 1).
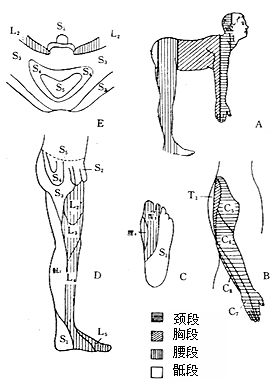
Figure 1 Trunk and limb skin sensory distribution map
A. Trunk and limbs B. Lateral aspect of the upper limb C. Sole of the foot D. Medial aspect of the lower limb E. Perineum
3. Sphincter dysfunction
Urinary and bowel dysfunction often occurs after motor and sensory impairments, initially presenting as difficulty urinating, with the urge but inability to urinate promptly, later developing into urinary retention. After the recovery of bladder reflex function, incontinence of urine occurs. Bowel dysfunction, in the initial stage [first stage], presents as abdominal distension and constipation, occasionally with diarrhea.
4. Autonomic dysfunction
Early paraplegia below the level of the lesion presents with dry skin without sweating. After the recovery of paraplegia, sweating function also recovers.
bubble_chart Auxiliary Examination
I. Imaging Studies
1. X-ray Imaging
Spinal anteroposterior and lateral radiographs showing paravertebral shadow enlargement and significant vertebral body destruction usually indicate the level of spinal cord compression. If the paravertebral abscess extends over 4 to 6 vertebral bodies, the level of bone compression should be determined in conjunction with signs, and if necessary, myelography, CT myelography (CTM), or MRI should be performed.
2. Myelography
Shows signs of extradural compression: The main feature is that the obstruction site on the anteroposterior view may appear brush-like or irregularly concave and convex, but without patchy filling defects. On the lateral view, the contrast medium at the compression site is displaced, increasing the distance from the bony spinal canal or showing filling defects. If the lesion is intradural, the contrast medium is not displaced, but the subarachnoid space shows thinning or discontinuity of the contrast medium, with patchy or small cup-shaped filling defects, or scattered small patchy distributions. Complete or partial obstruction does not significantly correlate with the degree of paralysis (complete or partial).
3. CT
CT is more valuable for locating small sequestrations causing compression.
4. MRI
In patients with severe paralysis, such as spastic flexion type, flaccid type, or healed lesion type, MRI is the preferred imaging method in addition to routine X-rays. It shows low signal intensity on T1-weighted images and relatively high signal intensity on T2-weighted images, revealing spinal abscesses and their extent of invasion into the spinal canal. Sagittal and axial views can accurately show the location of spinal cord compression by pus or granulation tissue. When images show more than 60% compression of the extradural space above the cauda equina, clinical examinations usually reveal varying degrees of spinal cord dysfunction.
MRI may occasionally show cystic changes in the spinal cord on T1-weighted sagittal images. In healed lesion-type paralysis, T1-weighted and T2-weighted sagittal images may show spinal cord thinning and atrophy at the most severe site of kyphosis, and occasionally, abnormal linear signals within the spinal cord itself on T1-weighted images (Figure 6, see insert).
II. Somatosensory Evoked Potentials (SEP) for Monitoring Spinal Cord Function
Over the past decade, SEP technology has been used to monitor spinal cord function during decompression surgery for traumatic or pathological paralysis and corrective surgery for scoliosis. During spinal surgery, the spinal cord may be subjected to varying degrees of compression, traction, vibration, or changes in blood perfusion, potentially leading to postoperative sensory and motor deficits. Therefore, intraoperative wake-up tests and/or SEP monitoring of spinal cord function are crucial for timely detection and management of issues. It is worth noting that intraoperative SEP monitoring may yield false negatives, so wake-up tests should also be performed simultaneously.
1. Intraoperative SEP Monitoring Methods
(1) Equipment: There are many types of instruments on the market. When selecting, pay attention to the following points: ① Compact size; ② Strong anti-interference capability; ③ Clear, stable, and reproducible signals; ④ Flexible software system for easy graphical analysis and measurement. {|113|} (2) Monitoring Parameters: Amplifier gain of 200,000 to 400,000 times, filter bandwidth of 1 to 1000 Hz; stimulator square pulse width of 0.1 to 0.5 ms, frequency of 2.5 times per second; stimulation intensity adjusted before anesthesia to elicit obvious dorsiflexion of the ankle joint. After anesthesia, due to the effects of muscle relaxants, the same level of electrical stimulation may not produce ankle movement, so the stimulation intensity should be appropriately increased. Current output can be set to 10 to 30 mA, and voltage output to 20 to 60 V. The stimulation intensity should not be too high to avoid nerve injury. Averaging 200 to 500 times, analysis time of 200 ms. In cases of spinal cord injury, SEP peak latency may be prolonged, sometimes exceeding 200 ms, which may be mistaken for SEP disappearance. Flexibility is required during monitoring.
(3) Stimulation and Recording Sites The negative pole of the stimulation is located 3 cm anterior and inferior to it. The recording site follows the standard 10/20 system of the International Federation of Clinical Neurophysiology, 2 cm posterior to the Cz point, with the reference electrode on one auricle. Both stimulation and recording electrodes are made of stainless steel needles.
2. SEP Spinal Cord Monitoring Indicators
Both the peak latency and amplitude of SEP can be used as monitoring indicators. It is generally believed that among the various waves, the P1 peak latency is relatively stable and consistent. The peak latencies of the SEP waves change from normal to abnormally delayed during surgery in the order of N2, P2, N1, P1, and the recovery order is antagonistic: P1, N1, P2, N2. However, there are also cases where only P1 shows abnormal changes.
When observing changes in amplitude, it is important to confirm that the electrode position, stimulation intensity, depth of anesthesia, and blood pressure remain unchanged. If the amplitude increases after decompression surgery compared to before, it is considered an improvement in function. If the spinal cord function and its amplitude are normal before surgery (Figure 1), but the amplitude decreases or increases during or after surgery, it may indicate an abnormal condition (Figure 2, Figure 3).
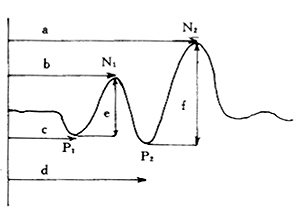
Figure 1 Normal Common Peroneal Nerve SEP
a. N2 peak latency b. N1 peak latency c. P1 peak latency d. P2 peak latency e. P1-N1 amplitude f. P2-N2 amplitude
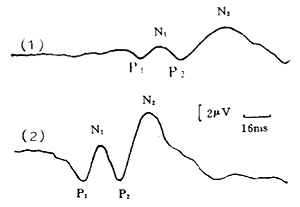
Figure 2 Common Peroneal Nerve SEP Monitoring During Thoracic Subcutaneous Node Paraplegia Surgery
(1) Preoperative prolonged peak latency, low amplitude
P1=75ms, N1=85ms
P2=98ms, N2=127ms
(2) Postoperative SEP returns to normal
P1=36ms, N1=49ms
P2=61ms, N2=81ms
Figure 3 Changes in Common Peroneal Nerve SEP Before and After T10~11 Subcutaneous Node Paraplegia Surgery
3. The Impact of Surgical Procedures on SEP
During decompression surgery for spinal subcutaneous node complicated by paraplegia, the impact of surgical procedures on the spinal cord, based on our intraoperative observations, can be summarized as follows: ① Caused by surgical vibrations or decompression of the spinal cord; ② In delayed onset paraplegia patients, intraoperative removal of bone to expose the spinal canal and extensive removal of surrounding bone may be related to the destruction of residual blood supply; ③ When rinsing the spinal cord with saline below 20℃, SEP changes or even disappears; ④ After intraoperative removal of extradural fibrous scar tissue, SEP changes significantly. Of course, some of the above reasons may coexist and collectively affect spinal cord function. Considering these factors, it is required that surgical procedures be accurate, gentle, and appropriately decompressed to achieve better surgical outcomes.
4. The Relationship Between Preoperative SEP Examination Results and Prognosis
According to examination data, preoperative examination of spinal subcutaneous node complicated by paraplegia shows that SEP is present in 93% of 86 cases of incomplete paraplegia, and SEP is not absent in 64% of 53 cases of complete paraplegia. This indicates that paraplegia caused by chronic compressive injury often results in incomplete spinal cord injury. There is a gradual transition between normal SEP and its disappearance, mainly manifested as prolonged peak latency and decreased or increased amplitude. Severe injury leads to prolonged peak latency, but we found no strict correspondence between peak latency and clinical signs.
In early compressive injury of the spinal cord due to spinal subcutaneous node complicated by paraplegia, prolonged peak latency is predominant. Patients with spinal subcutaneous node complicated by paraplegia whose SEP is absent during preoperative examination generally have a poor prognosis; conversely, the prognosis is better. Whether SEP appears or improves after spinal canal decompression is related to the duration of SEP absence before surgery. If surgery is performed within 1 to 3 weeks of SEP disappearance, SEP improvement can be observed during spinal canal decompression. Postoperatively, 87.5% of patients show varying degrees of functional recovery.
III. Lumbar Cerebrospinal Fluid Dynamics Test
Puncture site and nearby areas with infection or lumbar 1 subcutaneous node should not be performed.
1. Qeckenstedt Test
This is a method to check for obstruction in the subarachnoid space of the spinal canal. After a routine lumbar puncture, one person wraps a blood pressure cuff around the patient's neck, and another records. The operator connects the pressure measuring tube, measures the initial pressure, and records the height of the water column. Then, the assistant inflates the blood pressure cuff to 2.67 kPa (20 mmHg), and reports the pressure every 5 seconds until the pressure no longer rises. The assistant quickly deflates the cuff, and continues to report the pressure every 5 seconds until it returns to the original level or stops decreasing. Subsequently, the test is conducted with pressures of 5.33 kPa (44 mmHg) and 8.00 kPa (60 mmHg), and the results are recorded in the same manner. Finally, a curve is plotted based on the results.
(1) No obstruction in the subarachnoid space: The pressure rises to its peak about 15 seconds after neck compression and drops to the initial pressure level about 15 seconds after decompression. When compressed to 8 kPa (60 mmHg), it can rise to about 66.67 kPa (500 mmHg).
(2) Partial obstruction in the subarachnoid space: The rise and fall of cerebrospinal fluid pressure are slow after neck compression, or the rise is normal but the fall is slow, and the final pressure does not drop to the original level.
(3) No obstruction in the subarachnoid space: The pressure does not rise even when compressed to 8 kPa (600 mmHg).
Normal cerebrospinal fluid is colorless and transparent, with a cell count not exceeding 10, and protein content between 20% and 40 mg%. In cases of obstruction, the cerebrospinal fluid appears slightly yellow and transparent, with protein content increasing to several hundred milligrams%, while sugar and chloride levels are mostly normal, and the cell count does not change significantly. If the cell count also increases significantly, it may indicate subcutaneous node myelitis (Hodgson 1967).
2. Clinical Significance
If there is no obstruction before surgery, there is no need for decompression surgery. If there is obstruction before surgery and it is cleared after surgery, even if paraplegia does not recover, no further surgery is needed. If the obstruction remains after surgery and paraplegia does not recover, it indicates incomplete decompression, and further decompression surgery should be performed.
This test is simple and easy to perform, but according to Guan Hua's research data, it does not completely match with myelography, and myelography should be performed if necessary to verify.
Performing this test before, during, and after spinal canal decompression surgery, and comparing the results before and after, can monitor whether the decompression is satisfactory, thereby improving the surgical outcome.
1. The main function of the spinal cord is the control of the cerebral cortex over three functions: movement, sensation, and sphincter. It involves the transmission of sensations and the control of urination and defecation. Paraplegia is primarily characterized by dysfunction in active movement. Some scholars classify the degree of motor dysfunction in paraplegic patients into four levels to facilitate the observation of the progression of paraplegia during treatment and the evaluation of post-treatment outcomes.
Level I: The patient walks normally, feels strong in the lower limbs, and may or may not exhibit clonus upon examination. The metatarsus shows a positive pathological reflex.
Level II: The patient experiences muscle tension, spasms, weakness, and uncoordinated movements while walking. They may or may not require crutches to walk. Examination reveals spastic paresis in the limbs.
Level III: The patient is unable to walk due to muscle weakness in the lower limbs and is forced to stay in bed. Examination shows extensor-type paraplegia, with approximately 50% of cases exhibiting sensory disturbances.
Level IV: The patient exhibits flexor-type spastic paraplegia, with more than 50% of patients experiencing sensory disturbances. Bedsores are common, and there may also be sphincter dysfunction, including flaccid paralysis.
2. The Tianjin Paraplegia Index is divided according to the degree of loss of the three spinal cord functions, represented by indices 0, 1, and 2. 0 represents normal or near-normal function, 1 represents partial loss of function, and 2 represents complete or near-complete loss of function. The degree of loss in these three functions is not entirely parallel. It is common for the lower limbs to lose complete motor function while sensation and sphincter function remain intact. For ease of comparison before and after treatment, detailed records should be kept.
For example, if a patient has near-complete loss of motor function in the lower limbs, the index is 2. If sensation is dull but not completely lost, the total paraplegia index for the patient is 4. After treatment, if the patient fully recovers sphincter function and sensory disturbances but does not recover motor function, the total paraplegia index is 2. This indicates that the treatment plan is correct and effective and can be continued.
The paraplegia index has its advantages. Although it has fewer levels and provides only a rough representation of the degree of loss in the three functions, it remains a useful indicator.
3. Localization Diagnosis of Spinal Cord Compression: Determining the upper and lower boundaries of the lesion is usually not difficult. X-ray images showing severe vertebral destruction and paravertebral shadow enlargement can help localize the lesion. However, when X-ray images show paravertebral shadows extending over 4 to 6 vertebrae with no obvious vertebral destruction, a detailed neurological examination combined with other imaging results is necessary to determine the longitudinal level of compression.
(1) Determining the Upper Boundary of Spinal Cord Lesions: Radicular pain is of significant importance. Radicular pain is a direct manifestation of irritation of the sensory posterior root, characterized by dull pain, radiating pain along the nerve root. The radiation area generally corresponds to the distribution area of the affected root, often accompanied by cerebrospinal fluid impact pain (i.e., pain worsens with coughing, sneezing, or exertion).
After the resolution of spinal shock, reflexes can be used to determine the level of the lesion. The highest segment with absent reflexes may indicate the segment where the lesion exists (Figure 1).
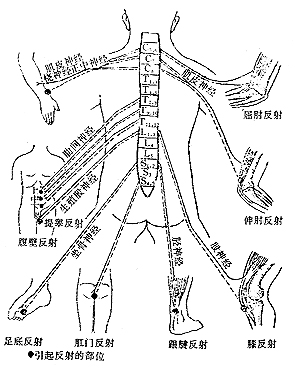
Figure 1: Various Reflexes and Their Relation to Spinal Levels
(2) Determining the Lower Boundary of Spinal Cord Lesions: Based on reflex changes, the highest segment with hyperreflexia can often infer the lower boundary of the lesion. For example, if a patient has diaphragmatic paralysis (C4) but hyperactive triceps reflex, it may indicate that the lesion involves C4 but not C5-6.
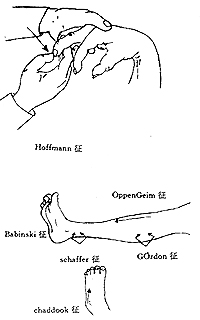
Figure 2: Pathological Reflexes
(3) Pathological Signs in Upper and Lower Limbs (Figure 2)
①Babinski sign: Use a blunt, pointed object to stimulate the outer edge of the patient's sole. In normal individuals, this causes flexion of the five toes at the metatarsus; in cases of pyramidal tract injury, dorsiflexion of the big toe occurs, with or without fanning of the other four toes, indicating a positive sign. In the vast majority of cases, this signifies a lesion in the pyramidal system, reflecting a disruption in the connection between the lower motor apparatus and the cerebral cortex.
②Chaddock's sign: Use a blunt object to stimulate the lateral edge of the dorsum of the foot, near the junction of the dorsum and the sole. The elicited reflex is the same as the Babinski sign, with similar sensitivity and significance.
③Oppenheim's sign: The examiner presses firmly on the front of the lower leg with the back of the thumb and index finger, moving downward. The elicited response is the same as the Babinski sign, which is dorsiflexion of the toes.
④Gordon's sign: The examiner tightly squeezes the gastrocnemius muscle, causing dorsiflexion of the big toe.
⑤Hoffmann's sign: The examiner supports the patient's wrist with the left hand, and with the right hand, the index and middle fingers grasp the patient's middle finger, and the thumb lightly flicks it, eliciting a reflex where the patient's thumb and other fingers have a flexion response.
bubble_chart Treatment Measures
Osteopathic active incomplete paraplegia can be treated with short-term non-surgical treatment. The majority of cases can recover. If there is no improvement, lesion clearance and spinal canal decompression can be performed. For osteopathic cured paraplegia, especially incomplete paralysis, non-surgical treatment is preferred initially. If there is no improvement, it is best to perform an MRI before surgery to clarify the location and extent of spinal cord compression and whether there are any lesions in the spinal cord itself. Surgical outcomes for osteopathic cured paraplegia with mechanical compression are generally poor. Surgery is best performed under the monitoring of somatosensory evoked potentials (SEP). Accurate decompression position, gentle surgical manipulation, and avoidance of vibration are required. Instruments should not press against the spinal cord. To maintain spinal stability and avoid injury to the main root stirred pulse, the range of spinal canal decompression should be moderate, and the scar tissue causing circular compression of the spinal cord should be removed.
I. Anterolateral spinal canal decompression
Initially proposed and designed by Capner, later improved by Alexander (1946) and Dott (1947).
1. Position
Lateral decubitus position, with the torso tilted forward at a 60° angle to the operating table. The side with the larger abscess and more severe paraplegia is chosen as the surgical side. The lower limbs are flexed at the hip by 45° and at the knee by 90°, with a soft pillow placed between the legs. The knee joints and pelvis are secured with straps to maintain the position.
2. Anesthesia
Endotracheal intubation general anesthesia.
3. Procedure
(1) Incision: On the surgical side of the back, along the spine, centered on the affected vertebra, make a curved or straight incision. The apex of the curved incision is 8 cm from the midline, and the incision length is 12-14 cm (Figure 1).
Figure 1: Anterolateral spinal canal decompression (Capner) incision
(2) Surgery: Incise the skin, subcutaneous tissue, superficial and deep fascia, then sequentially incise the first layer of trapezius and latissimus dorsi muscles, and the second layer of rhomboid and serratus posterior muscles along the incision direction, and retract them laterally. At 4-5 cm from the spinous process, where the sacrospinalis muscle is thinner, make a longitudinal incision, and retract the muscle bilaterally to expose the proximal ends of 2-3 ribs corresponding to the affected vertebra that need to be removed. Dissect the periosteum along the circumference of the rib connected to the affected vertebra until the rib neck and transverse process, and cut the rib at the neck. Cut the other end of the rib 6 cm lateral to the transverse process, remove the rib, and use a round-headed periosteal elevator to strip the inner and lower periosteum along the rib, taking care not to tear the pleura. Fully expose the rib head, use the transverse process as a fulcrum, and pry out the rib. At this time, pus may be seen oozing out, which should be suctioned. Remove the upper and lower ribs in the same manner. Along the space between the rib bed and pleura, bluntly push the pleura forward to expand the space until reaching the affected vertebra and the front of the spine. Scrape out the contents of the lesion, caseous material, and dead bone.
Free the intercostal nerve and protect it, ligate the intercostal vessels, use the intercostal nerve as a guide to locate the intervertebral foramen, and enlarge it with a small Kerrison rongeur. Then remove the pedicle to expose the lateral aspect of the spinal canal, where the spinal cord can be seen. Similarly, remove the head and neck of the upper and lower ribs and the pedicle. The number of ribs to be removed depends on the extent of the lesion, usually 2-3. At the posterior aspect of the affected vertebra, i.e., the anterolateral aspect of the spinal canal, gently scrape and cut the caseous material, dead bone, or necrotic disc compressing the spinal cord, taking care not to touch the spinal cord. The granulation tissue surrounding the spinal cord dura or the fibrous scar causing circular compression should also be dissected and removed. If the affected vertebra severely protrudes posteriorly and compresses the spinal cord, part of the protruding bone ridge can be removed to achieve adequate spinal cord decompression. After decompression, the surface of the spinal cord (in cured lesions) can be covered with free fat grafts to prevent scar formation and re-compression of the spinal cord.
During spinal cord decompression, try to preserve the transverse processes of the vertebral body. The lamina and its upper and lower articular processes should not be removed, as this will affect spinal stability (Figure 2).
Figure 2 Anterolateral Decompression of the Spinal Canal (Capner) Decompression Range
II. Transthoracic Lesion Clearance Combined with Anterolateral Decompression of the Spinal Canal
1. Anesthesia
Thoracic Spine 2~3~4 Subcutaneous node or Thoracic Spine 11~12 Subcutaneous node Patients adopt improper means to obtain bronchial intubation general anesthesia. During surgery, the lung on the operative side collapses, providing a spacious surgical field for easier manipulation.
2. Position
Lateral decubitus position, with the torso and both upper limbs fixed as before.
3. Procedure
(1)Incision A posterolateral thoracic incision centered on the thoracic lesion, selecting an appropriate level for the incision. For example, in cases of thoracic 7~9 Subcutaneous node with paraplegia, enter the chest by removing the 7th or 8th rib on the side with severe paraplegia and large paravertebral abscess; for thoracic 10~11~12 Subcutaneous node, remove the 9th or 10th rib to enter the chest and perform lesion clearance and spinal canal decompression.
(2)Surgery The steps and methods for exposing the thoracic lesion are detailed in relevant chapters.
III. Anterolateral Decompression of the Spinal Canal
On the lateral side of the affected vertebral body, make a transverse incision along the direction of the severed rib head and neck through the parietal pleura, intersecting perpendicularly with the longitudinal incision for paravertebral abscess clearance to form a T-shape (Figure 3).

① ②
Figure 3 Transthoracic Lesion Clearance Combined with Anterolateral Decompression of the Spinal Canal
① Schematic diagram of transthoracic lesion clearance and anterolateral decompression 1. Lesion clearance 2. Spinal canal decompression 3. Prevertebral abscess 4. Right lung 5. Left lung
② Position of the T-shaped incision for spinal canal decompression and paravertebral approach
From the transverse incision, dissect the rib stump and rib head and remove the pedicles of the upper and lower vertebrae. During this process, do not remove the transverse process, superior and inferior articular processes, or lamina on the operative side to maintain spinal stability.
The method for clearing substances compressing the spinal cord, such as subcutaneous nodes, is the same as described in previous relevant chapters and will not be repeated here.
Depending on the extent of vertebral destruction, use the resected rib or harvest an iliac bone graft for interbody fusion to reconstruct spinal stability. After thorough hemostasis of the spinal lesion, irrigate the T-shaped paravertebral incision and suture it in layers with interrupted silk sutures. Place a closed drainage tube at the 7th or 8th intercostal space along the posterior axillary line on the operative side, and close the chest cavity in layers.
Postoperative management: Continue anti-subcutaneous node medication and administer anti-infective drugs. For patients with high-level paraplegia and intercostal muscle paralysis leading to ineffective sputum expulsion, assist in sputum expulsion to prevent respiratory infections or atelectasis. Keep the closed drainage unobstructed to avoid pleural effusion, and manage abdominal distension and fullness to prevent affecting pulmonary ventilation.
1. Better prognosis: Younger patients, shorter duration of paraplegia, active lesions, extensor type paraplegia, slow progression of paraplegia, and spinal cord compression due to sexually transmitted disease have a better prognosis.
2. Poor prognosis: Generally poor condition, elderly patients, longer duration of paraplegia, healed lesions, complete flexor spastic type, rapid progression of paraplegia, flaccid paraplegia, kyphotic deformity greater than 60° (Tuli 1993) (Figure 1), combined with subcutaneous node disease in other areas or major organ diseases.
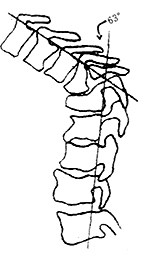
Figure 1: Kyphotic deformity exceeding 60°
3. Time of neurological recovery after surgery
According to clinical observations, the fastest recovery begins 24 hours after decompression surgery, with 90% of cases starting recovery within 6 weeks post-surgery. Tuli (1993) reported recovery starting between 24 hours and 12 weeks post-surgery; most patients began recovery 3 weeks after surgery. The degree and duration of paraplegia showed no significant correlation with the speed of postoperative recovery. Complete recovery typically takes 3 to 6 months. Hong Kong (1998) reported 22 cases of delayed onset paraplegia, with an average recovery time of 6.8 months for active lesions and up to 24 months for healed lesions.
Pattisom (1993) reported 89 cases treated with chemotherapy alone (non-surgical). Before treatment, paraplegia persisted for less than 3 months in 48% of cases, 3 to 6 months in 27%, 6 to 12 months in 4 cases, and over 12 months in 2 cases. After treatment, recovery occurred within 3 months in 42 cases (48%), 3 to 6 months in 27 cases (30%), and the remaining 4 cases took 6 to 12 months.
4. Sequence of spinal cord neurological recovery
First, vibration sense and joint position sense, followed by temperature, touch, and pain sensation, then autonomic function, sphincter function, and muscle atrophy.
According to domestic data, complete recovery of paraplegia after surgery occurs in about 89% of cases. Tuli (1975–1985) reported complete recovery in 72%, walking with crutches in 11%, and 7% died from various complications. Patients with kyphotic deformity greater than 60° mostly achieved partial recovery. Notably, urinary tract infections and severe bedsores are the main causes of death in paraplegic patients and should be prevented.
1. Neurogenic Bladder Dysfunction
The micturition function of the bladder requires close coordination between the detrusor muscle and the urethral sphincter. After spinal cord injury, the brain and sacral cord centers lose control over the detrusor muscle and urethral sphincter, meaning the central nervous system cannot control the micturition function, collectively referred to as neurogenic bladder dysfunction. Patients often suffer from urinary tract infections due to impaired or lost micturition function.
1. Classification
Previously, micturition dysfunction was classified into autonomous bladder and reflex bladder types. Recently, a more detailed classification of neurogenic bladder based on the detrusor function has been proposed:
(1) Detrusor hyperreflexia, further divided based on sphincter function:
① Normal sphincter coordination, presenting with urgency and frequency.
② External sphincter dyssynergia, presenting as urinary retention.
③ Internal sphincter dyssynergia, presenting as urinary retention.
(2) Detrusor areflexia
① Normal sphincter coordination, presenting as urinary retention.
② External sphincter spasm, presenting as urinary retention.
③ Internal sphincter spasm, presenting as urinary retention.
④ External sphincter denervation (relaxation), presenting as incontinence.
2. Clinical Manifestations
In cases of detrusor hyperreflexia with normal sphincter coordination, clinical manifestations include urgency and frequency. Most patients initially present with urinary retention. Except for patients with sphincter denervation (relaxation) who present with incontinence, other patients, regardless of detrusor strength, cannot urinate due to uncoordinated internal and external sphincters. When the bladder accumulates a large amount of urine, its internal pressure exceeds the tension of the sphincter, causing urine to overflow. In the late stage [third stage], sphincter relaxation, especially in those with long-term indwelling catheters, presents as incontinence.
3. Management
In the initial stage [first stage] of paraplegic patients, renal failure caused by infection and/or obstruction remains the main cause of death in paraplegic patients with neurogenic bladder dysfunction. The main goal of conservative therapy is to restore the balance function of the bladder and urethra and to prevent infection. Various non-surgical treatment methods include: ① Indwelling catheterization; ② Intermittent catheterization; ③ External urine collection devices; ④ Drug therapy, etc. Sterile intermittent catheterization is the preferred treatment method.
(1) Intermittent Catheterization Most researchers believe that the advantages of using intermittent catheterization include: ① Reducing the rate of urinary tract infections to about 50%; ② Allowing patients to urinate on their own when there is no bladder outlet obstruction; ③ Promoting early recovery of bladder function through regular bladder expansion; ④ Fewer penile and scrotal complications.
The patient's daily fluid intake is strictly controlled at 1500-1800 mL. Depending on the amount of urine, the patient is catheterized 3-4 times within 24 hours to maintain a bladder capacity below 500-600 mL.
Before spontaneous urination occurs, catheterization is performed every 6 hours. When spontaneous urination and reduced residual urine occur, catheterization can be performed every 9 hours, then every 12 hours. Catheterization can be stopped when residual urine is less than 100 mL. Various auxiliary methods are used to promote urination before each catheterization. Urine tests and cultures are performed weekly. If signs and symptoms of sepsis appear, appropriate antibacterial drugs should be administered.
Contraindications for intermittent catheterization include: ① Urethral deformity; ② Severe urethritis; ③ Perurethral abscess.
For patients performing self-catheterization at home and in places lacking personnel and equipment for sterile intermittent catheterization, this method is used with the same indications and precautions as sterile catheterization. Disposable sterilized catheters that are more affordable for patients can be used.
Operation: The female patient washes her hands and perineum with soap and water. The patient assumes a semi-recumbent position with thighs flexed and knees abducted to expose the vagina and urethral orifice, allowing the patient to view her perineum in a mirror placed at the foot of the examination table. The labia are separated, and the patient is shown the location of the labia, urethral orifice, and vagina. The patient is given a 14F plastic or rubber catheter and instructed to insert it into the urethral orifice and advance it into the bladder.
The guidance for male self-catheterization is relatively simple. The patient should sit, apply water-soluble lubricant to the catheter, use one hand to retract the foreskin, and use the other hand to insert the catheter into the urethral orifice until urine flows out.
(2) Indwelling Catheterization
1) Indications ① Incomplete bladder emptying, complete urinary retention, or incontinence in critically ill and debilitated patients; ② Suitable for intermittent catheterization but unable to perform it. ③ Upper urinary tract damage or certain neurogenic bladder dysfunctions with vesicoureteral reflux.
2) Precautions for Indwelling Catheterization ① The insertion should be gentle, using more lubricant formula; ② Choose the smallest catheter size that fits the patient's urethral orifice; ③ Use a closed drainage system; ④ Place the drainage bag below the bladder level to prevent urine backflow; ⑤ Clean the external urethral orifice and catheter exit with cotton balls soaked in antibiotic solution 2-3 times daily; ⑥ Ensure adequate fluid intake; ⑦ Use 4% boric acid solution or 0.2% nitrofurazone solution for irrigation.
3) Complications of Indwelling Catheterization ① High incidence of urinary tract infections; ② Bladder stones; ③ Chronic contractile bladder infections; ④ Penile and scrotal complications: abscess, urethral fistula, and epididymitis; ⑤ Hematuria; ⑥ Limb paralysis, catheter blockage, urinary retention, and autonomic reflex dysfunction.
II. Bedsore
1. Grading of Bedsore
Local skin redness and hardening are Grade I; epidermal purplish-red with blisters not reaching the subcutaneous layer is Grade II; bedsore reaching the subcutaneous tissue, sometimes exposing muscle or tendon is Grade III; local tissue necrosis reaching the bone is Grade IV.
2. Common Sites of Bedsore
Below the level of paraplegia, where skin sensation is lost, the skin over bony prominences is prone to bedsore. Common sites when lying supine are the sacrum, both greater trochanters, and the scapular region; when lying prone for a long time, the anterior superior iliac spine and the front of the patella may develop bedsore.
3. Prevention and Treatment
Laying a sponge mattress on the bed or placing sponge pads at bony prominences, and turning the patient every 3-4 hours to avoid prolonged pressure on bony prominences are prerequisites for preventing and treating bedsore. Small Grade I and II sores can gradually heal with local dressing changes. Small Grade III and IV sores leave scars after healing; larger sores, if conditions permit, should be repaired with skin or musculocutaneous flaps.
III. Bowel Dysfunction
Bowel dysfunction in paraplegic patients often manifests as constipation. Studies show that the peristaltic sequence of the ascending colon, transverse colon, descending colon, and sigmoid colon in these patients is no different from that in normal individuals. The cause of constipation is the uncoordinated action of the anal sphincter, which remains tense during defecation. For such cases, anal suppositories are preferable to laxatives or manual removal of stool.
Patients with constipation often experience abdominal distension and fullness, especially those with higher levels of paralysis, causing significant discomfort. Relief of constipation improves abdominal distension and fullness.





