| disease | Pancreatic Cancer (Hepatobiliary Surgery) |
Pancreatic cancer is highly malignant, difficult to detect early, metastasizes early and rapidly, and has a poor prognosis. In recent years, the incidence of pancreatic cancer has increased both domestically and internationally. According to statistics from Peking Union Medical College Hospital and the Affiliated Hospital of Hunan Medical College in China, the number of hospitalized patients with pancreatic cancer in the past decade has increased by 3 to 4 times compared to the previous 10 years. In the United States, the incidence of pancreatic cancer has tripled, ranking fourth in cancer mortality, following lung cancer, colorectal cancer, and breast cancer, and is comparable to prostate cancer. In Japan, the incidence has quadrupled, while in the UK and Germany, it has doubled. In 1986, the number of new cases diagnosed with pancreatic cancer in the United States reached 25,550, with 24,000 deaths from the disease. Pancreatic cancer is more common in males and typically occurs in individuals over 40 years old, with the head of the pancreas being the most frequent site (70-80%). It can be multicentric or spread directly within the pancreas. This disease is one of the most challenging gastrointestinal tumors to diagnose early. Clinically, only 10-30% of advanced pancreatic cancer cases can be radically treated, and the survival rate remains very low post-treatment, with a 5-year survival rate of 0.18-5%. Specifically, the 3 and 5-year survival rates for pancreatic head cancer are the lowest among gastrointestinal cancers. To improve the survival rate of pancreatic cancer, the key is to enhance early clinical detection rates.
bubble_chart Pathological Changes
1. Clinical Pathology and StagingPancreatic cancer is a malignant tumor arising from the exocrine tissue of the pancreas. Most originate from ductal epithelium, while a minority arise from acinar epithelium. Pancreatic cancer accounts for 1-4% of all cancers.
1. Gross Findings:
Pancreatic cancer occurs in the head of the pancreas in 70-80% of cases, in the body and tail in 20-30%, and diffusely throughout the pancreas in a minority. Tumors often protrude from the pancreatic surface, forming hard or coarse nodules, grayish-white in color, with often indistinct margins. Tumors vary in size; small adenocarcinomas may be embedded within the pancreatic parenchyma, but the surrounding pancreatic tissue often becomes sclerotic, and sometimes the pancreas may be deformed. Pancreatic cancer can sometimes be difficult to distinguish from chronic pancreatitis, leading to misdiagnosis of chronic pancreatitis as pancreatic cancer and subsequent radical surgery. Carcinoma of the pancreatic head often invades the duodenal wall, blurring the normal relationship with the ampulla, but the duodenal mucosa is generally still normal. The cut surface of the tumor is grayish-white and hard, with a minority being gelatinous, papillary, or cystic and softer; if there is hemorrhage and necrosis, it may also become soft.
2. Microscopic Findings:
Based on histological morphology, it can be classified into adenocarcinoma, adenosquamous carcinoma, mucinous adenocarcinoma, cystadenocarcinoma and papillary cystadenocarcinoma, giant cell carcinoma, acinar cell carcinoma, and undifferentiated carcinoma, among others.
(1) Adenocarcinoma: Also known as ductal cell adenocarcinoma or columnar cell carcinoma, it is the most common type, accounting for about 90%. Cancer cells are mostly tall columnar or cuboidal, with round or oval nuclei mostly located at the base. The cytoplasm is often water-clear, without granules, and occasionally cilia are seen. Cancer cells form irregularly sized and shaped glandular lumens, which may be dilated but not cystic. Some adenocarcinomas have small and uniform nests, resembling small glandular structures, and are often positive for mucin staining.
(2) Mucinous Adenocarcinoma: Also known as colloid carcinoma, it is relatively rare. The glandular lumens of adenocarcinoma are significantly enlarged and contain a large amount of mucus. Sometimes, a large number of "signet-ring cells" can be seen, floating in the mucus or appearing in clusters.(3) Cystadenocarcinoma and Papillary Cystadenocarcinoma: They account for a small portion of adenocarcinomas. The nests of adenocarcinoma expand into multiple cystic cavities, with cancer cells on the cavity walls growing papillary into the lumen, even filling the cavity.
(4) Undifferentiated Carcinoma: The cells are small, with scanty cytoplasm, small nuclei, spindle-shaped or round, and prominent nucleoli. Cancer cells often diffusely infiltrate in sheets, with some arranged in nests or cords resembling simple carcinoma. Mucin staining is often negative.
Others: Acinar cell carcinoma, giant cell carcinoma, adenosquamous carcinoma, etc., are even rarer.
The above types may sometimes appear in the same cancerous tissue, constituting a mixed type of pancreatic cancer.
In the normal tissue surrounding pancreatic cancer, goblet cell hyperplasia and hypertrophy of ductal epithelium, hyperplasia of mucous glands, or squamous metaplasia can be seen; papillary hyperplasia and atypical hyperplasia of ductal epithelium, even forming carcinoma in situ and early invasive carcinoma, are particularly common in adenocarcinoma, indicating that pancreatic cancer originates from ductal epithelium. Pancreatic cancer is often accompanied by extensive thrombosis; cancer tissue not only invades local portal veins, mesenteric veins, splenic veins, etc., forming thrombi, but can also form thrombi elsewhere in the body.
3. Biological Characteristics of Pancreatic Cancer:Pancreatic ductal carcinoma is closely related to pancreatic ductal epithelial hyperplasia, and the coexistence of pancreatic ductal carcinoma and pancreatic ductal epithelial hyperplasia has been observed in some surgically resected specimens. Therefore, pancreatic ductal epithelial hyperplasia may be a precancerous lesion of pancreatic cancer. Pour et al. reported that the incidence of pancreatic ductal epithelial hyperplasia is 57%, while the incidence of pancreatic cancer is 10%. Furuta classified pancreatic ductal carcinoma into two types: mass-forming type and duct-expanding type. They can occur in the main pancreatic duct, branch pancreatic ducts, and terminal branches of the pancreatic ducts and acini. Mass-forming pancreatic cancer mainly occurs at the branch sites of the pancreatic ducts, predominantly as tubular adenocarcinoma. Duct-expanding pancreatic cancer is more common in the main pancreatic duct, primarily as papillary carcinoma, accompanied by a large amount of mucus, and diffusely grows within the duct. Mass-forming pancreatic ductal carcinoma grows from the periphery toward the central main pancreatic duct, thus causing dilation of the main pancreatic duct due to compression or obstruction by the enlarging tumor. Duct-expanding pancreatic ductal carcinoma occurs in the main pancreatic duct or adjacent branch pancreatic ducts, and early-stage narrowing of the main pancreatic duct is common, leading to dilation of the distal main pancreatic duct. Since this type of cancer secretes a large amount of mucus, tumor cells are prone to spread within the duct. Duct-expanding pancreatic cancer is easier to detect early through imaging, and its prognosis is better than that of the mass-forming type. In surgically resected specimens, the majority of duct-expanding pancreatic cancers are confined within the pancreas.
Another biological characteristic of pancreatic cancer is that even if it is a small pancreatic cancer, the cancer cells have already infiltrated and metastasized to the surrounding areas through lymphatic vessels, surrounding nerves, loose and connective tissues. Often, before cancer cells invade the lymph nodes, there is already infiltration of surrounding nerves and lymphatic vessels.
4. Metastasis of Pancreatic Cancer:
How pancreatic cancer metastasizes and through what pathways has been debated for years without a consensus.
One opinion suggests that pancreatic cancer early on causes peribiliary infiltration, with pancreatic head cancer often invading the common bile duct early, even if the pancreatic cancer is small (diameter <2cm) and at a considerable distance from the common bile duct, significant peribiliary infiltration can occur. This is not due to the direct involvement of the lower end of the bile duct by adjacent cancer tissue, but rather the metastatic infiltration of pancreatic head cancer, possibly through lymphatic spread within the pancreatic head to the wall of the common bile duct. Whether pancreatic body cancer spreading to the posterior abdominal membrane could have the same nature is considered highly possible.
Another viewpoint suggests that pancreatic cancer is multicentric from the start within the pancreas. Given this argument, total pancreatectomy should be performed regardless of the location of the pancreatic cancer. Motojima's study using PCR method on K-ras gene mutations suggests that multicentric occurrence exists but is very low, only 6%, thus it is not necessary to perform total pancreatectomy on all pancreatic cancers.
The metastasis pattern of pancreatic ductal carcinoma is from the pancreatic duct to surrounding nerves to lymphatic vessels and finally to blood vessels. The characteristic of lymph node metastasis in pancreatic cancer is that metastasis is more common in the upper group of the pancreatic head and the posterior group of the pancreaticoduodenal lymph nodes; the metastasis rate in the upper group of the pancreatic body and the lower group of the pancreatic head is next, with fewer lymph node metastases found in the splenic hilum area, posterior to the common bile duct, and around the stomach. Although early pancreatic cancer has infiltration of adjacent lymphatic vessels, surrounding nerves, and loose connective tissues, lymph node metastasis is not necessarily present. Nagai reported that pancreatic cancer at 0.4×0.3cm already had pathological infiltration of lymphatic vessels, surrounding nerves, and adjacent loose connective tissues, but no peripancreatic lymph node metastasis was found. The blood route metastasis of pancreatic cancer first occurs in the proximal bleeding vessel area near the cancer growth, and when the cancer mass is small, it may invade the portal vein and stirred pulse. Tashiro reported that even if pancreatic cancer is judged to have invaded the portal vein macroscopically, 25% still show no histological infiltration, and although histologically the outer membrane or middle membrane of the vessel is invaded, 51.9% show no inner membrane infiltration. Therefore, even if a small pancreatic cancer invades the portal vein, surgical resection should not be abandoned. However, in the early surgical resection of pancreatic cancer, one should not only be satisfied with local tumor resection but should also remove adjacent connective tissues, surrounding abdominal nerves, and lymph nodes together, theoretically making the surgery thorough.
5. Clinical Staging of Pancreatic Cancer:
There are many staging standards for pancreatic cancer, each with its representativeness and reflecting its characteristics, but the key issue is still the presence or absence of lymph node metastasis. Currently, there is no universal classification method, and several commonly used classification methods are introduced below:
1. Hermreck's Staging Method (1974)
Stage I: Tumor is limited to the pancreas
Stage II: Tumor only invades adjacent tissues, such as the duodenal wall
Stage III: There is already regional lymph node metastasis
Stage IV: There is already liver and other distant metastasis
2. Kloppol's TNM Staging (1979)
T 1 : Tumor is limited to the pancreas
T 2 : Tumor has extended beyond the pancreas
T 3 : Tumor has invaded adjacent organs (duodenum, stomach, and spleen)
N 0: No lymph node metastasis
N 1 : Regional lymph node metastasis around the pancreas
N2: Metastasis in adjacent regional lymph nodes
M0: No hematogenous metastasis
M1: Hematogenous metastasis
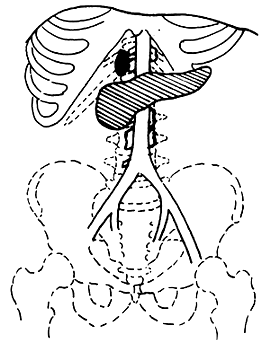
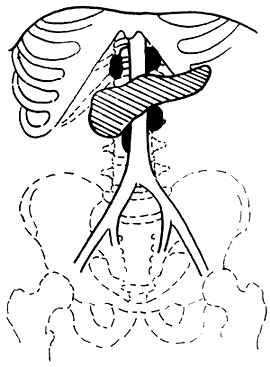
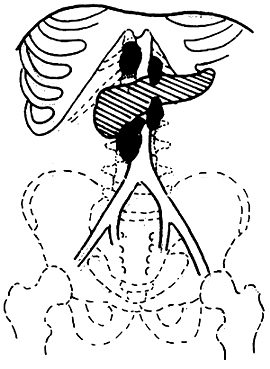
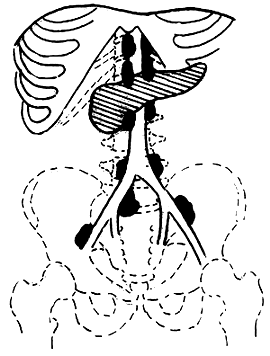
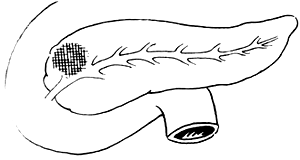
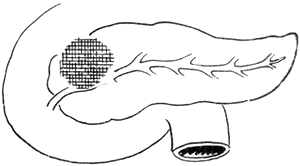
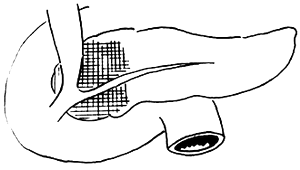
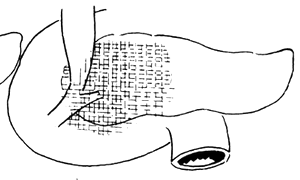
3. Adama's revised anatomical staging (1983) (Figure 1)
T1
 >
>
T2
 >
>
T3
 >
>
T4
 >
>
N1
 >
>
N2
 >
>
N3
 >
>
N4
Figure 1 Anatomical staging of pancreatic cancer
T1: <2cm, confined to the pancreas
T2: 2-6cm, confined to the pancreas
T3: >6cm
T4: Invasion of adjacent tissues outside the pancreas
N0: No lymph node metastasis
N1: Metastasis to only one regional lymph node during laparotomy
N2: Invasion of two regional lymph nodes
N3: Clinically palpable regional lymph node metastasis
N4: Distant regional lymph node metastasis
4. Qian Li's classification of pancreatic head cancer (1989)
Stage I: T1N0M
0T1: Tumor within the pancreas, solitary, generally <2cm
N1: No lymph node metastasis
M0: No vascular metastasis
Stage II: T2N1M
0T2: Cancer has extended beyond the pancreatic capsule, generally <5cm, invading adjacent tissues, bile duct or duodenum, or multiple lesions in the bile duct
N1: Metastasis to the first station lymph nodes
Stage III: T2-3N1-2M
0T3: Pancreatic cancer has invaded the stomach, spleen, or retroperitoneal tissues such as PV or SMV, generally >5cm
N2: Second station lymph node metastasis
Stage IV: T2-3N1-3M1
T4: Pancreatic cancer has adhered to surrounding tissues
N3: Metastasis to the third station lymph nodes
M1: Liver metastasis or other distant metastases
bubble_chart Clinical Manifestations
Pancreatic cancer often lacks specific symptoms in its early stages. Early manifestations include upper abdominal discomfort, often presenting as dull pain, distending pain, or aching pain. Symptoms worsen after meals, possibly due to varying degrees of bile duct obstruction. After meals, especially after high-fat meals, bile and pancreatic juice secretion increases, leading to increased pressure. When pancreatic cancer is located in the tail of the pancreas, early symptoms are particularly subtle, with only discomfort and distending pain in the upper left abdomen. As pancreatic cancer progresses to the middle and advanced stages, symptoms become more pronounced: jaundice gradually appears and progressively worsens, leading to deep jaundice. Stools also gradually turn clay-colored, accompanied by symptoms such as cutaneous pruritus due to hyperbilirubinemia. As the disease progresses further, severe pain radiates to the lower back, making it difficult to sleep day or night, caused by the cancer invading the celiac plexus. Tumors in the tail of the pancreas can compress the splenic vein, leading to splenomegaly and severe pain in the left flank. Sometimes, cancer emboli invade the splenic vein and portal vein, causing regional portal hypertension, followed by hypoalbuminemia and the appearance of significant ascites. In the advanced stages of pancreatic cancer, signs become evident: deep jaundice, hepatomegaly to the point where the gallbladder can be palpated, and a palpable, tender, and fixed mass in the upper abdomen.
bubble_chart Auxiliary Examination
I. Measurement of Tumor-Associated Antigens
The use of monoclonal antibody technology has led to the discovery of many new tumor-associated antigens, some of which have high sensitivity for pancreatic cancer.
1.CA19-9 and CA50: Normal pancreatic tissue can express CA19-9 and CA50. Histochemistry shows that CA50 is present in small terminal ducts and central acinar tissues, with the strongest staining in large ducts, while CA19-9 is rarely stained in small ducts. Normal islet cells do not express CA19-9 or CA50. Clinical findings indicate a high correlation between serum antigen concentration and pancreatic tumor size. The detection rate of CA19-9 for pancreas cancer is comparable to CT. Using a threshold of 37μ/ml, Sakahara reported positive rates of CA19-9 in cases with tumor diameters of <3cm, 3-5cm, and >5cm as 13%, 90%, and 92% respectively.
Alberto used solid-phase radioimmunoassay to detect CA19-9, setting a standard of 40μ/ml, with a sensitivity of 90% for diagnosing pancreatic cancer. Farini used thresholds of 17μ/ml and 37μ/ml for CA19-9, examining 130 cases of pancreatic cancer and chronic pancreatitis, with diagnostic sensitivities of 86.7% and 62.3%, and specificities of 73.3% and 87.0% respectively. Sakahara, using a standard of CA19-9 at 37μ/ml, examined 55 cases of pancreatic cancer and 22 cases of chronic pancreatitis, with an accuracy of 83%. Some scholars believe that when CA19-9 is <200μ/ml, the likelihood of resection is high, and patients whose CA19-9 levels return to normal after tumor resection have a better prognosis. A postoperative rise in CA19-9 indicates tumor recurrence or metastasis, but CA19-9 can also rise during acute episodes of chronic pancreatitis.
Using CA19-9 as a screening antigen is significant, with a cutoff value of 120μ/ml. A value above 120μ/ml highly suggests pancreatic cancer, with a positive rate of 85% in the general population. In populations with jaundice, the positive predictive value is 100%.
2.SPan-1: SPan-1 is a monoclonal antibody produced by immunizing Balb/c mice with a cell system of human pancreatic cancer that produces mucin. Satake reported that the sensitivity, specificity, and accuracy of SPan-1 for diagnosing pancreatic cancer were 81.4%, 67.5%, and 71.1% respectively. Some scholars have reported that the sensitivity of SPan-1 can reach up to 100%, with a specificity as high as 82.9%.
3. Mucin glycoproteins: Mucin glycoproteins are frequently found in the serum of pancreatic cancer patients. Parker developed an enzyme-linked complex (CAM17.1/WGA), which, when added to the serum being tested, can detect the presence of mucin glycoproteins, thereby diagnosing pancreatic cancer. Using CAM17.1/WGA alone to diagnose pancreatic cancer, the sensitivity is 78%, and the specificity is 76%. When combined with CA19-9, the sensitivity can be increased to 85%, while the specificity remains unchanged.
II. Ras Gene Testing
K-ras, H-ras, and N-ras are common oncogenes found in human tumors. The mutation rate of ras genes varies among different tumors, with the highest mutation rate observed in pancreatic cancer. The mutation rate of the K-ras gene is approximately 90%. In pancreatic cancer, K-ras gene mutations predominantly occur at codon 12. The mutation rate of codon 12 in the K-ras gene varies during the progression of pancreatic cancer: 26% in the hyperplasia stage, 46% in the papillary hyperplasia stage, 76% in the carcinoma in situ stage, 80% in the pancreas cancer stage, and 43% in the lymph node metastasis stage. The mutation of K-ras is not significantly related to tumor staging, but the mutation frequency is higher in stage I and II tumors compared to stage III and IV tumors. This mutation is an initial triggering factor and does not participate in the late-stage [third stage] development of the tumor. In pancreatic tumors, different mutations in the K-ras gene within the same tumor increase the likelihood of multifocal tumor origin. The increase in the mutation rate of the K-ras gene is significantly correlated with tumor size, with smaller tumors having a significantly lower mutation rate than larger tumors. The mutation rate of the K-ras gene in pancreatic cancer is much higher than that in ampullary cancer. The potential of using K-ras gene mutations for detecting pancreatic cancer requires further exploration.
III. Imaging and Cytological Examination
1. B-mode Ultrasound Imaging: Based on the gross pathological features and structure of pancreatic cancer, the following manifestations can be observed on ultrasound images:
(1) Hypoechoic Type: Due to the lower echo level of the tumor compared to normal tissue, the boundary between the lesion and surrounding pancreatic tissue is relatively clear, showing irregular, jagged, or pseudopod-like extensions. The lesion area exhibits scattered, unevenly distributed hypoechoic regions, with grade I enhancement or no significant change in the posterior and distal echoes.
(2) Heterogeneous Echo Type: The lesion primarily shows low to medium-level echoes, interspersed with coarse high-echo spots or densely clustered small masses. The boundary with surrounding tissue is relatively clear but often irregular, with some areas appearing blurred. Due to significant ultrasound attenuation, the posterior and distal echoes may show grade I weakening. This type of ultrasound image often occurs when the cancer has hemorrhage, necrosis, or abundant connective tissue.
(3) Anechoic Type: The lesion area shows no echoes or only a few scattered low-echo spots. The posterior and distal echoes may exhibit enhancement. This type of ultrasound image is rare and occurs when the cancerous tissue has a gelatinous appearance.
(4) Mixed Echo Type: Part of the lesion area shows low to medium echoes, while another part shows high echoes, forming unevenly distributed spots of varying thickness. Anechoic areas of different shapes may also coexist. The boundary between the cancer and surrounding tissue is relatively clear. This type of ultrasound image often occurs when there is internal hemorrhage, blood accumulation, or liquefactive necrosis within the tumor.
(5) Changes in the Main Pancreatic Duct: Depending on the tumor's location, the main pancreatic duct may be compressed, leading to dilation, displacement, twisting, interruption, or a beaded appearance. Tumors close to the main pancreatic duct may compress the duct wall, causing an arc-shaped indentation or displacement. When the main pancreatic duct is invaded by cancer, the duct wall becomes irregular.
(6) Changes in Bile Ducts and Blood Vessels: Tumors in the pancreatic head compress the lower end of the bile duct, causing varying degrees of dilation in the biliary system above the compression. As the tumor grows, signs of compression may appear in the adjacent proximal vessels, such as indentation, displacement, or flattening of the portal vein and inferior vena cava.
2. Due to the different locations of pancreatic cancer, the ultrasound images vary accordingly.
(1) Ultrasound Image of Pancreatic Head Cancer: The pancreatic head is enlarged and thickened, with an irregular shape. A pancreatic head diameter >4 cm often indicates a space-occupying lesion. The common bile duct is dilated, the gallbladder is enlarged, and uniform echoes of biliary sludge may be observed. The intrahepatic bile ducts are also dilated. The main pancreatic duct, portal vein, and inferior vena cava may show signs of compression.
(2) Sonographic images of pancreatic body cancer: The pancreatic body is significantly thickened with irregular protrusions; the tumor area shows hypoechoic echoes, and the boundary between the tumor and the surrounding pancreatic tissue is sometimes blurred and sometimes clear; the main pancreatic duct and abdominal aorta, as well as the inferior vena cava, show signs of compression. If the splenic vein is compressed, the spleen may exhibit congestive enlargement. Upon re-examination after drinking water, compression of the gastric antrum and gastric angle can be observed, with the stomach being pushed forward and upward.
(3) Sonographic images of pancreatic tail cancer: Fasting detection of the pancreatic tail is often difficult to display. It can be detected through the "acoustic window of the stomach" after drinking water, or through the spleen from the intercostal space, or through the kidneys from the back in a prone position; after drinking water, if the lesion is on the lesser curvature side, the stomach can be seen pushed forward and to the left; when the lesion is near the greater curvature, the gastric body can be seen pushed to the right.
Pasanen reported that the sensitivity, specificity, and efficiency of ultrasound in diagnosing pancreatic cancer were 61.9%, 93.9%, and 91.6%, respectively. The sensitivity is significantly lower than that of CT and ERCP. The false negatives in ultrasound are mainly due to unsatisfactory display, and ultrasound is also more difficult in diagnosing tumors <2cm, with a sensitivity of only 55%. If ultrasound is combined with tumor markers, the sensitivity of each can be significantly improved, compensating for the shortcomings of using ultrasound alone. In recent years, endoscopic ultrasound (EUS) has been used to diagnose pancreatic cancer, which is a technology combining endoscopy and ultrasound, aiming to improve the detection rate of pancreatic cancer. However, its practical value still needs to be concluded through continuous practice.
3. CT scan imaging: The diagnostic value of CT for pancreatic cancer seems similar to that of B-ultrasound in early studies, but in recent years, the combination of plain and enhanced CT scans has provided greater diagnostic value for pancreatic cancer. An important image in CT scanning is: pancreatic enlargement and the disappearance of peripancreatic fat spaces; it can also show the location, size, density of the mass, whether the pancreatic duct is dilated, and whether blood vessels are invaded. Pancreatic cancer CT manifestations include: enlargement of the pancreatic head, lobulated shape, and destruction of the normal smooth curve of the boundary. If the tumor liquefies and necroses, it can show low-density shadows within the tumor. In pancreatic head cancer, the body and tail of the pancreas are often accompanied by edema or atrophy. Tumors in the uncinate process of the pancreas are easily overlooked in the early stages. If the uncinate process loses its unique shape, with forward or backward rounded protrusions extending behind the superior mesenteric vein, it suggests the presence of a tumor in this area. Another image of uncinate process tumors is: it will lift the superior mesenteric vein from behind, showing elongation and straightening of the superior mesenteric vein on cross-section. Due to local infiltration of the cancer, the peripancreatic fat spaces can be interrupted, destroyed, or even disappear. Indirect signs of pancreatic cancer include varying degrees of dilation of the pancreatic duct and common bile duct. Zeiss conducted a retrospective analysis of enhanced CT in 56 cases of pancreatic cancer and compared it with surgical and Freeny's research results, concluding that CT has high accuracy in judging the unresectability of tumors, especially with the use of dynamic CT, with a reliability of 100%; but the accuracy in judging resectability is poor. Because CT cannot show subtle pancreatic invasion and surface metastatic lesions. Pasanen reported that the sensitivity, specificity, and efficiency of CT were 95.2%, 92.9%, and 96.6%, respectively. However, CT has a sensitivity of only 77% in diagnosing tumors <2cm.
4. Magnetic Resonance Imaging (MRI): MRI is superior to CT and B-ultrasound in detecting small tumors confined within the pancreas and determining the presence of peripancreatic spread. The diagnostic signs of pancreatic cancer on MRI are: on T1-weighted images, there is a localized signal decrease within the pancreatic parenchyma; on T2-weighted images, the density ratio between the tumor and normal tissue is low, which often limits its use; if T1-weighted images show a reticular low to medium signal in the fat behind the abdominal membrane, it can be diagnosed as early local invasion; invasion of the peripancreatic area, behind the pancreas, stomach, duodenum, adrenal glands, common bile duct, and hepatic hilum indicates severe local invasion and unresectability. Studies combining MRI and pathological examinations have shown that in T1-weighted images, a localized signal decrease within the pancreatic parenchyma accompanied by areas of signal enhancement corresponds to the parenchymal part, while areas of signal decrease show significant hemorrhage and necrosis.
5. Endoscopic Retrograde Cholangiopancreatography (ERCP): ERCP examination can directly observe lesions in the pancreatic duct on one hand, and on the other hand, obtain specimens through biopsy and brushing for a definitive pathological diagnosis. Therefore, its diagnostic accuracy can be over 90%. About 80% of pancreatic cancers are ductal epithelial carcinomas, mostly located in the pancreatic head, thus showing involvement of the pancreatic head. The pancreatic duct may show varying degrees of stenosis, interruption, or displacement, with the stenotic segment often feeling rigid. If the tumor in the pancreatic head is large, the pancreatic duct may appear very short or obstructed. In cases of incomplete obstruction, significant dilation distal to the obstruction can be seen. When there is an irregular protrusion or brush-like change in the pancreatic duct, it may indicate communication with necrotic cancerous lesions or fistula formation. When pancreas cancer mainly invades the pancreatic head parenchyma, the pancreatic duct often appears displaced or encroached upon. If the bile duct in the pancreatic head segment is involved, a "double duct sign" or rigidity, irregularity, or even twisting of the common bile duct may appear. Some classify pancreatic cancer into five types: stenotic, occlusive, pancreatic parenchymal defect, intraductal growth, and compressive types. Small pancreas cancers often manifest as stenotic, occlusive, or pancreatic field defect types. Currently, ERCP still has difficulty diagnosing small cancers that have not invaded the pancreatic duct. The changes in the pancreatic duct between pancreatic cancer and chronic pancreatitis should be differentiated. In chronic pancreatitis, the main pancreatic duct and secondary branches show irregular twisting, stenosis, or alternating dilation, known as a beaded appearance. In some cases of recurrent episodes, multiple cystic dilations may be seen, and some may show calcifications. Direct magnification radiography of the pancreatic duct can help differentiate pancreas cancer from chronic pancreatitis.
Although ERCP has a high diagnostic accuracy for pancreatic cancer, most cases detected have already lost the opportunity for radical surgery.
6. Percutaneous Transhepatic Cholangiography (PTC): We began using PTC for biliary diseases in the 1950s. At that time, we used thick needles for puncture without a TV-equipped X-ray machine, which was quite challenging, but it provided significant diagnostic value for complex biliary diseases. Since the 1970s, we have used fine needles for puncture under the guidance of a TV-equipped X-ray machine, with almost no failures. To prevent complications such as biliary fistula and peritonitis after PTC in patients with deep jaundice, we generally perform PTC on the operating table during surgery, followed immediately by the surgical procedure, thus minimizing complications. Among the 358 cases of fine-needle PTC we have performed, only one case developed peritonitis requiring emergency surgery. PTC is an excellent method for diagnosing biliary diseases such as bile duct cancer and benign stenosis. PTC not only excludes biliary lesions but also reveals indirect signs of pancreatic head cancer compressing or invading the pancreatic segment of the bile duct, such as bile duct compression, displacement, and rigidity. Bakkevdd summarized the results of 472 patients, showing that PTC has a sensitivity of 85% for diagnosing pancreatic cancer and 78% for stage I lesions. Some have used PTCD for drainage to alleviate jaundice after PTC in patients with deep jaundice. However, we believe that preoperative PTCD increases the risk of infection and generally do not recommend it.
7. Fine-Needle Aspiration Cytology (FNA): Using FNA under CT or ultrasound guidance for diagnosing pancreatic cancer has significantly improved diagnostic accuracy. FNA can be performed percutaneously before surgery or directly during surgery. Although percutaneous FNA passes through some organs, complications are rare if the technique is properly mastered. The issue of tumor seeding along the needle tract is also very rare. In 1981, Beazlcy reported a positive rate of 87-100% for FNA. In 1988, Warshaw concluded that the accuracy of fine-needle aspiration cytology is 87% with no false positives.
8. Cytological examination of pancreatic juice: Pancreatic juice is obtained through fiberoptic duodenoscopy for cytological examination, but the false-negative rate is high. Inserting the endoscope directly into the pancreatic duct to aspirate specimens for cytological diagnosis, especially for pancreatic body and tail cancer, has higher diagnostic accuracy. If a tube is placed during surgery to collect pancreatic juice, precise localization diagnosis of occult pancreas cancer can be made. Some have used this method to detect in situ pancreas cancer.
9. Selective Angiography (SAG): The early signs and characteristics of SAG in pancreatic cancer are tumor infiltration into blood vessels. The earliest blood vessels infiltrated by pancreatic head cancer are the duodenal stirred pulse and its branches; pancreatic body or tail cancer can infiltrate the dorsal pancreatic stirred pulse, greater pancreatic stirred pulse, tail pancreatic stirred pulse, and transverse pancreatic stirred pulse. SAG can display the aforementioned stirred pulses and visualize the celiac stirred pulse surrounding the upper part of the pancreas; catheter insertion into the superior mesenteric stirred pulse can display the blood vessels surrounding the lower part of the pancreas. Catheter insertion into the gastroduodenal stirred pulse and common hepatic stirred pulse can display the stirred pulses of the pancreatic head; insertion into the splenic stirred pulse can display the pancreatic body and tail. Changes in these blood vessels are used to diagnose pancreatic cancer. Blood vessels infiltrated by cancer appear stiff, narrowed, or blocked, with irregular vessel wall contours. If major blood vessels such as the hepatic stirred pulse, celiac stirred pulse, or main trunk of the superior mesenteric stirred pulse are invaded, it indicates that the disease has reached an advanced stage and is unresectable. Changes in the blood vessels of pancreatic cancer itself can be observed through super-washed stirred pulse angiography, revealing fine, tortuous, and irregular blood vessels within the tumor.
SAG has high accuracy in diagnosing tumors <2cm, and its capillary phase can show the invasion and blockage of intra-pancreatic blood vessels caused by small tumors, as well as the corresponding hypovascular area in the tumor region. If the tumor is confined to the ductal epithelium and not accompanied by pancreatic parenchymal invasion, SAG cannot detect it.
With the advancement of medical technology, the methods for detecting pancreatic cancer have increased, providing a basis for early detection of the disease. Given that the initial symptoms of pancreatic cancer are vague, most patients have already lost the opportunity for surgery by the time they seek medical attention, and the prognosis is poor. Therefore, in regions with the necessary conditions, regular physical examinations should be conducted, including imaging techniques such as ultrasound, CT, MRI, ERCP, and SAG, as well as cytological examination of pancreatic fluid, cytological examination of pancreatic puncture tissue, measurement of cancer-related antigens, and ras gene testing. These methods have significantly improved the early detection rate of pancreatic cancer.
bubble_chart Treatment Measures
1. Current Status of Pancreatic Cancer Surgery Treatment
The treatment of pancreatic cancer still faces a series of issues to date. Therefore, it is difficult to unify its treatment with a single therapeutic approach. Many factors influence the treatment: the biological characteristics of pancreatic cancer differ from those of ampullary cancer, with early metastasis, some cases being multicentric, and others involving periductal infiltration around the distal common bile duct; the location, size, and malignancy of pancreatic cancer all affect the outcome of surgery, as reported by Laurent (1993) that the efficacy of radical pancreatic cancer cases is constrained by many factors (Table 1); there is considerable debate over surgical methods, whether to adopt radical Whipple surgery, total pancreatectomy, or extended pancreatic head cancer resection, which has not been fully unified. The clearance range of radical Whipple surgery is insufficient and needs to be expanded, while total pancreatectomy has not shown significantly better resection rates, surgical mortality, and 3- and 5-year survival rates compared to Whipple, and the postoperative endocrine and exocrine functions will depend on external sources for life; extended Whipple can preserve part of the pancreatic tail, thus maintaining endocrine function, but the invaded vessels around the pancreas cancer also need to be removed, not only is the surgical trauma large, but the 1- and 3-year cure rates still need to be supported by a large number of cases; for patients with deep jaundice, whether preoperative PTCD drainage decompression is necessary has not been fully agreed upon. The above issues have been perplexing surgeons, sometimes making it difficult to determine the treatment plan.
Table 1 1-year and 5-year survival rates and influencing factors after radical pancreatic resection
| Factor | 1-year (%) | 5-year (%) |
| Location | ||
| Pancreas | 58.5 | 10.4 |
| Periampullary | 78.6 | 52.4 |
| Tumor Grade | ||
| I or II | 75.0 | 53.6 |
| III or IV | 63.6 | 18.5 |
| Bilirubin | ||
| <2 | 85.7 | 71.4 |
| >2 | 62.4 | 18.0 |
| Differentiation | ||
| Good | 90.0 | 20.2 |
| Moderate or Poor | 50.0 | 0.0 |
| Size | ||
| <4cm | 67.7 | 23.7 |
| >4cm | 61.4 | 40.9 |
| Duodenal invasion | ||
| Yes | 71.6 | 59.7 |
| No | 64.0 | 15.5 |
| Lymphatic stagnancy of yang | ||
| None | 53.2 | 44.4 |
| One or more | 78.6 | 26.6 |
| Radiotherapy | ||
| Yes | 72.9 | 0.0 |
| No | 68.3 | 35.7 |
II. Surgical Treatment of Pancreatic Cancer
(1) Pancreatic cancer is an extremely debilitating disease
that causes significant physiological disturbances. The main pathophysiological changes in pancreatic cancer include: jaundice and its resulting coagulation disorders; grade III emaciation and malnutrition leading to a series of systemic changes; and endocrine changes such as hyperglycemia or hypoglycemia.
1. Jaundice: Progressive worsening is a prominent symptom of pancreatic cancer. When the bile and pancreatic ducts are blocked, it affects the digestion and absorption of fats, leading to vitamin deficiencies and a lack of vitamin K-dependent clotting factors (prothrombin, factors VII, IX, and X). Long-term bile duct obstruction can cause liver damage and also lead to deficiencies in other non-vitamin K-dependent clotting factors (such as factor V), and can easily cause fibrinolysis, resulting in extensive bleeding in the surgical field. After vitamin K injection, when the prothrombin time returns to normal or near normal, bleeding can be significantly reduced during surgery. Another serious issue with severe obstructive jaundice is the high risk of acute renal insufficiency after surgery. The causes of acute renal insufficiency in severe jaundice are multifaceted: such as low renal perfusion and glomerular filtration rate; jaundice increases the kidney's susceptibility to hypotension and hypoxia. Hyperbilirubinemia itself can cause severe damage to the glomeruli and renal tubules. Therefore, for patients with a clear diagnosis of periampullary carcinoma, after proper preoperative correction of systemic conditions, early surgery should be performed, and close attention should be paid to water and electrolyte balance postoperatively.
2. Emaciation: Emaciation is another prominent manifestation of pancreatic cancer. The causes of emaciation are often due to insufficient intake, persistent pain, and impaired digestive and absorptive functions. Prolonged insufficient protein intake leads to the depletion of protein components in the body such as muscles, myocardium, enzymes, and antibody proteins, making these patients more susceptible to postoperative infections, poor wound healing, wound dehiscence, and other complications. As tissues are consumed, body weight rapidly decreases, and the total blood volume in the body also decreases, along with a reduction in red blood cell volume. The body then produces a series of adaptive responses, such as systemic vascular bed contraction, to adapt to the reduced blood volume and maintain blood pressure at a certain level. However, in such conditions, anesthesia and surgery can lead to additional blood loss, fluid loss, and a sharp reduction in circulating blood volume, causing blood pressure to drop rapidly and even leading to shock. Therefore, it is extremely important to actively improve the patient's nutritional status and enhance their immune capacity before surgery to reduce surgical mortality and postoperative complications. For example, short-term preoperative intravenous nutritional support or elemental diet (about 2 weeks) can improve the patient's overall condition and reverse negative nitrogen balance.
3. Changes in endocrine function: Patients with pancreatic diseases may suffer from hyperglycemia or hypoglycemia. For surgical patients, both high and low blood sugar levels are dangerous. Factors that can cause high or low blood sugar before and after surgery include anesthesia, trauma, increased secretion of catecholamines, infusion of hypertonic glucose, fasting, and excessive use of insulin. Typically, it is advisable to maintain postoperative urine sugar levels at + to ++ for diabetic patients to avoid the risks of hyperglycemia or hypoglycemia.
Therefore, preoperative correction should be actively pursued to achieve a positive balance. This includes supplementation of water, electrolytes, vitamins K and C, clotting factors, and albumin.
Currently, there is considerable debate regarding the management of deep jaundice: one approach is to perform PTCD drainage to alleviate symptoms before surgery, while another argues against PTCD drainage.
Nakayama reported that for patients with bilirubin levels exceeding 171μmol/L, PTCD (PTBC) was performed on 108 cases, reducing mortality from 28.3% to 8.2-6%. Braasch noted that mortality was 22% for patients with bilirubin levels above 342μmol/L, and 13% for those below this level. Denning reported no postoperative complication deaths for patients with bilirubin levels below 102.6μmol/L, and that preoperative PTCD reduced surgical mortality from 25% to 16%, and postoperative complications from 56% to 28%. Pitt et al. found no difference in mortality between the PTCD group and the control group (8.1% and 5.3%, respectively), while Pherson et al. (1984) reported higher mortality in the PTCD group compared to the control group (32% vs. 19%). The PTCD group also had higher rates of bleeding and infection complications and longer hospital stays.
The 1988 National Symposium on Pancreatic Diseases concluded: "For malignant obstructive jaundice, whether to perform preoperative drainage (such as PTCD) cannot be generalized. If the disease course is short and the patient's condition is not severely compromised, and it is estimated that the patient can tolerate surgery, the difference in outcomes with or without preoperative drainage is minimal, and routine drainage is generally not performed"; "Because PTCD carries a high risk of complications (bleeding, infection, tube dislodgement, biliary fistula disease, etc.), once complications occur, the opportunity for effective resection is often lost. If hyperbilirubinemia has already affected renal function, drainage should be considered to protect the kidneys."
In our experience with malignant obstructive jaundice, the majority of cases did not undergo preoperative drainage. Surgery was performed under active preoperative preparation. If PTC examination was performed, it was generally done the day before or on the day of surgery. When performed the day before surgery, accumulated bile was aspirated as much as possible after the procedure, generally preventing biliary fistula disease.
(2) Pancreaticoduodenectomy (Whipple/Child surgery)
The surgical method proposed by Whipple in 1935 for treating periampullary cancer was later refined by Child and has been used for over half a century. Unfortunately, the Whipple procedure has unsatisfactory resection rates and postoperative outcomes for pancreatic head cancer, partly due to the biological characteristics of pancreatic head cancer and partly due to the limitations of this surgical approach. Consequently, total pancreatectomy and regional pancreatectomy have been used to treat pancreatic cancer (discussed further below).
Although the Whipple procedure is commonly used, several issues need to be discussed:
1. Post-Whipple pancreatic endocrine and exocrine function
Most of the islet cells are distributed in the tail of the pancreas, followed by the body of the pancreas, with the least in the head of the pancreas. The scope of the Whipple procedure includes the head of the pancreas and the pancreatic tissue on the right side of the mesentery. The tail and most of the body of the pancreas are preserved, but whether diabetes will occur is uncertain. Some reports indicate that about 15% of patients will eventually develop diabetes after this surgery. Yasugi et al. reported that if 85-90% of the pancreas is removed, almost 100% of patients will develop diabetes; if 75-85% is removed, delayed diabetes will occur 8-12 weeks postoperatively; and if less than 70% is removed, diabetes will not occur. In a typical Whipple procedure, if less than 70% of the pancreas is removed, diabetes will not occur. In our cases (45 cases), no endocrine dysfunction of the pancreas has been observed. Whether exocrine function is insufficient still depends on: the amount of residual pancreas; the management of residual pancreatic cancer. If 50% of the pancreatic tissue is preserved, and the pancreatic duct is correctly anastomosed with the jejunum without stenosis, the exocrine function of the residual pancreas can be maintained.
2. Common Diagnostic Errors During Surgery
Misdiagnoses of pancreatic head cancer and ampullary cancer during surgery occur frequently and must be taken seriously.
Pancreatic Head Cancer: It is often mistaken for chronic pancreatitis, or chronic pancreatitis may be misdiagnosed as pancreatic head cancer. Differentiating between the two is often challenging. There have been reports of cases where chronic pancreatitis was misdiagnosed as pancreatic head cancer, leading to unnecessary pancreaticoduodenectomy. Sometimes, pancreatic head cancer coexists with inflammation, especially when the tumor is embedded within a chronically inflamed and enlarged pancreatic head, making diagnosis particularly difficult. Key points for differential diagnosis include: in chronic pancreatitis, the pancreas often shows uniform enlargement with surrounding inflammatory edema. In pancreatic head cancer, the mass is usually very hard. When the pancreatic duct is obstructed, it often appears dilated, with little inflammatory exudate around the pancreas.
To confirm the diagnosis, fine-needle aspiration biopsy can be performed during surgery, avoiding tissue biopsy as much as possible. If pancreatic tissue is excised, it should be done carefully. Shallow excisions often yield no positive findings, while deep or large excisions, even if sutured, can lead to complications such as pancreatic fistula.
Ampullary Cancer: Generally, after fully mobilizing the duodenum, a preliminary diagnosis can be made through palpation. If the common bile duct is opened and palpated from within, it provides further diagnostic evidence.
However, it is important to clarify the following before resection:
(1) What is the nature of the mass? Benign or malignant?
(2) Is it a stone granuloma or a tumor? In rare cases, stones impacted in the ampulla can cause chronic irritation, leading to the proliferation of granulation tissue around the stone, which may be mistaken for a tumor.
Given that pancreaticoduodenectomy is a highly destructive procedure, in addition to the two palpation methods mentioned above, the duodenum should be opened to directly observe changes in the duodenal papilla. Typical signs include a cauliflower-like protrusion of the papilla that bleeds easily upon touch, warranting a frozen section examination. If the disease presents atypically, the sphincter of Oddi should be incised for direct visualization. If stones and fibrous granulation tissue are found together, a frozen section should also be performed to rule out malignant transformation due to chronic inflammation.
3. Comprehensive Exploration
Before performing pancreaticoduodenectomy, a thorough intra-abdominal exploration must be conducted. There have been instances where distant metastases were discovered only after the surgery had begun or progressed to a certain stage, leaving the surgeon in a dilemma. Therefore, before deciding on pancreaticoduodenectomy, the following sequence of examinations must be performed.
Full Abdominal Exploration: The exploration should be systematic, typically starting with the liver, hepatoduodenal ligament, root of the small intestine, transverse mesocolon, and pelvis. If no metastases are found, further exploration is conducted.
Exploration Around the Pancreas: This step is crucial for the success of the surgery. The gastrocolic ligament is incised along the transverse colon, and the stomach is retracted to examine the surface of the pancreas, the surrounding peripancreatic area, the upper and lower edges of the pancreas, and the area around the abdominal aorta for any metastatic lesions. If no signs of metastasis are found, proceed to the next step.
The lateral peritoneal membrane of the duodenum is incised to fully mobilize the pancreatic head, primarily checking for adhesions and cancerous infiltration between the pancreatic head and the inferior vena cava.
If the previous step shows no abnormalities, further exploration is conducted to check for cancerous infiltration between the pancreatic neck and the portal vein, as well as the superior mesenteric artery and vein. The common bile duct is first transected (Figure 1). When transecting the common bile duct, it should be done as close to the upper edge of the duodenum as possible. Subsequently, the right gastric artery and gastroduodenal artery are ligated and transected. The pyloric antrum is retracted downward, and gentle blunt dissection is performed on the dorsal side of the pancreatic neck. If there is no adhesion or tumor infiltration between the pancreatic neck and the portal vein, the same method is used to dissect the lower edge of the pancreas to check for adhesions between the pancreatic neck and the superior mesenteric vein. If the upper and lower dissections meet without any adhesions, it indicates no adhesions are present.
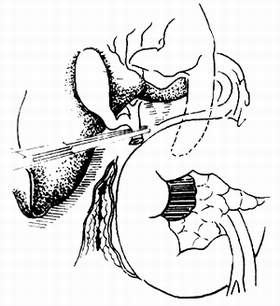
(1) After transecting the common bile duct, check for tumor infiltration in the portal vein.
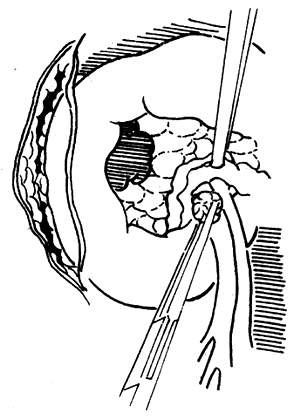
(2) Exploring the relationship between the tumor and the veins on the mesenteric membrane.
Figure 1: Exploring the relationship between the pancreas and its surroundings.
If any adhesion or cancer infiltration is found during the above steps, radical surgery should not be performed hastily. Otherwise, it may lead to a dilemma during the operation or cause massive bleeding.
4. Several issues during the pancreaticoduodenectomy procedure.
Common mistakes when cutting the pancreas: It is incorrect to use a sharp instrument to cut the pancreatic neck in one go. This often results in the pancreatic duct being cut and retracted, making it extremely difficult to locate. Instead, a combination of sharp and blunt dissection should be used, with intermittent suturing as the cutting progresses. When approaching the pancreatic duct area, careful dissection is essential, and the pancreas should not be completely cut blindly. Generally, the pancreatic duct is dilated and located at the junction of the upper and middle third of the pancreatic projection (Figure 2). Finding a thinner pancreatic duct can be quite challenging, but with careful and meticulous separation, it can usually be located. If it retracts, it becomes difficult to find.
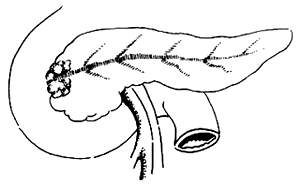
Figure 2: The location of the pancreatic duct at the junction of the upper and middle third of the pancreatic projection.
The length of the freed pancreatic duct should be more than 3mm. Before cutting, a small hole should be made and a catheter inserted as a marker. After cutting, two 5-0 sutures should be placed for traction (Figure 3). The pancreatic stump should be sutured in two layers. When suturing near the pancreatic duct, the tension should be moderate to avoid compressing the duct.
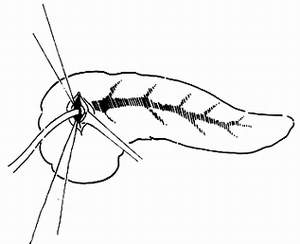
Figure 3: Suturing the pancreatic duct with two stitches and inserting a catheter.
Common mistakes during the dissection of the dorsal side of the pancreatic head: The main mistake during the dissection of the dorsal side of the pancreatic head is improper handling of blood vessels. The dorsal side of the pancreatic head is connected to the portal vein, mesenteric vessels, and the celiac plexus. Carelessness during the dissection can lead to bleeding. Particularly, the small branches that drain into the mesenteric vein from the dorsal side of the pancreatic head, usually 3 to 6 in number, have thin and fragile walls and should not be clamped. Instead, they should be ligated with 3-0 silk sutures before being cut (Figure 4). After properly handling these small veins, the mesenteric vein and the pancreatic head can be separated, and the vein should be carefully pulled to the left. Behind it lies the mesenteric artery. The connective tissue should be cut, and the arteries entering the uncinate process of the pancreas should be ligated. At this point, only a thick layer of the celiac plexus remains connected to the dorsal side of the pancreatic head, which should be cut near the pancreatic head (Figure 5).
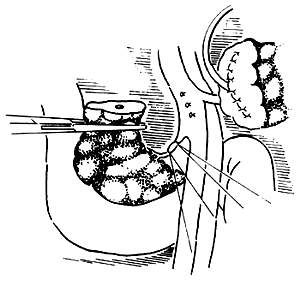
Figure 4: Ligating the small veins draining into the mesenteric vein from the pancreatic head.
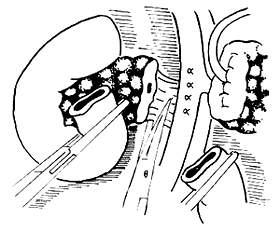
Figure 5: Cutting the nerve plexus on the dorsal side of the pancreatic head.
Resection of the stomach, the lower end of the common bile duct, the pancreatic head, the duodenum, and part of the jejunum (5-10cm distal to the ligament of Treitz). The posterior abdominal wall in the upper abdomen will have a large wound surface with significant exudation, so thorough hemostasis is essential. It is easy to overlook the protection of the stump of the gastroduodenal artery. Before reconstructing the gastrointestinal, pancreaticojejunal, and biliary-jejunal anastomoses, the greater omentum should be used to cover the stump of the artery and the mesenteric vessels to prevent pancreatic juice leakage and massive bleeding.
Example: Zhang ××, male, 59 years old. Progressive jaundice for 2 months, accompanied by pain in the upper right abdomen as the jaundice deepened. Diagnosis of periampullary carcinoma was made through examinations such as duodenal hypotonic radiography. Exploratory laparotomy was performed under general anesthesia. The liver was enlarged due to cholestasis, and the bile ducts were dilated. After careful examination and frozen section biopsy of the papilla, the diagnosis was papillary carcinoma of the ampulla. A conventional pancreaticoduodenectomy was performed. The gastric duodenal stirred pulse stump was ligated and sutured through. The pancreaticojejunostomy was performed using the end-to-end invagination method with drainage. The postoperative course was stable, but on the 7th day after surgery, the patient suddenly developed a pale complexion, and blood pressure dropped to 6.67/0 kPa. A large amount of fresh blood flowed from the abdominal drainage site, and the abdomen subsequently became distended. Under pressure transfusion, rapid entry into the abdominal cavity revealed it filled with blood clots. After clearing the blood clots, examination around the pancreaticojejunostomy revealed that the bleeding originated from the gastric duodenal stirred pulse stump. This stump and its surrounding area had been eroded by pancreatic enzymes. The common hepatic stirred pulse and the proper hepatic stirred pulse were then ligated, and the bleeding stopped. A plasma tube and thin membrane latex tube were placed behind the pancreas for drainage. Postoperatively, a small amount of pancreatic fluid drained from the tube, and the drainage tube was gradually removed after 10 days, with good recovery.
Should the gallbladder be removed? Given that the biliary-enteric anastomosis results in the loss of function of the Oddi's sphincter, it is difficult to maintain the normal pressure within the biliary tract, leading to poor gallbladder emptying. Long-term accumulation of bile can cause chronic cholecystitis, hence the gallbladder should be removed.
5. Several issues regarding the reconstruction of the stomach, biliary tract, pancreas, and jejunum
The principle that alkaline-secreting organs should be positioned above should be followed. To neutralize gastric acid and reduce the occurrence of anastomotic ulcers, the order of reconstruction with the intestine should be biliary-jejunal anastomosis, pancreatic-jejunal anastomosis, and gastro-jejunal anastomosis.
The methods for pancreatic-jejunal anastomosis can include end-to-side pancreatic-jejunal anastomosis and end-to-end invagination of the pancreas into the jejunum (Figure 6). Generally, the end-to-end invagination method is not preferred as it has a higher rate of postoperative pancreatic fistula complications compared to the former. Therefore, it should only be used when the pancreatic duct is too narrow to anastomose properly.
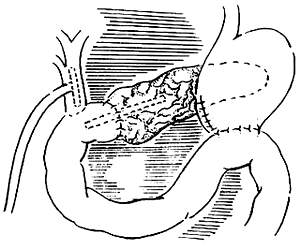
(1)
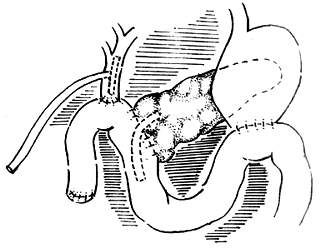
(2)
Figure 6 Two types of anastomosis
End-to-side biliary-jejunal anastomosis. The diameter of the bile duct is generally large, making the anastomosis usually not difficult. To prevent the reflux of gastric and pancreatic juices from affecting the healing of the biliary-enteric anastomosis, a T-tube should be placed above the anastomosis for external drainage.
The reason for the failure of end-to-side pancreatic-jejunal anastomosis is the failure to achieve a proper anastomosis between the pancreatic duct and the jejunal mucosa. The number of sutures depends on the diameter of the pancreatic duct; for smaller ducts, there should be no fewer than four sutures, otherwise pancreatic fistula may occur. To prevent anastomotic stenosis and to direct pancreatic juice into the intestinal tract, a pediatric urinary catheter or a silicone tube of corresponding thickness should be placed into the pancreatic duct and pulled out through a hole in the intestine (Figure 7). The depth of the support drainage tube in the pancreatic duct should be such that after the catheter is inserted into the pancreatic duct to the maximum depth, it is then withdrawn by 1-2 cm.
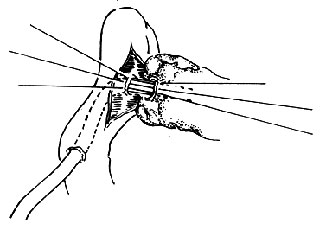
Figure 7 Support within the pancreatic duct pulled out through the jejunum
Gastro-jejunal anastomosis is performed routinely, but it is important to note the distance between the anastomosis and the biliary and pancreatic-jejunal anastomosis, which should generally be at least 20 cm below these two anastomoses. This is to prevent reflux biliary and pancreatic ductitis.
6. Placement of drainage tubes
Given the large wound surface and significant bleeding after duodenectomy, especially the leakage of pancreatic juice causing fistula disease, which can corrode and digest surrounding organs leading to major bleeding and intestinal fistula, it is crucial to correctly select and properly place drainage tubes.
(1) Principles for selecting drainage tubes: soft, unobstructed drainage, minimal irritation, and tube holes not easily blocked by fibrin.
(2) Placement of drainage tubes: It is advisable to place a thin latex tube and a cigarette drain near the biliary anastomosis. Behind (dorsal to) the pancreatic-jejunal anastomosis, place a cigarette drain. Five drainage tubes should be properly marked and pulled out of the body along with the T-tube. Each drainage tube must be pulled out through a separate hole in the abdominal wall (Figure 8). The size of the hole should be such that it can accommodate a finger next to the drainage tube under anesthesia. Drainage tubes must never be pulled out through the abdominal wall incision, otherwise pancreatic juice may seep into the layers of the abdominal wall, causing digestion and incision dehiscence.
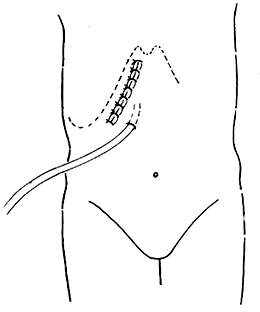
Figure 8 Drainage tubes pulled out through separate holes in the abdominal wall
(3) Since the pancreaticojejunostomy is a critical step in the Whipple procedure, improper handling can lead to a series of complications, such as pancreatic fistula disease, hemorrhage, and even complete surgical failure. Particularly, if pancreatic fluid is not drained externally, the activation of pancreatic proenzymes can cause digestion around the fistula disease, resulting in extensive tissue necrosis, hemorrhage, and even complete dissolution of the anastomosis. We once encountered a case where improper drainage of the pancreaticojejunal anastomotic fistula disease led to the digestion and rupture of the gastroduodenal stirred pulse, causing severe massive hemorrhage. Summarizing the current management of the pancreatic duct:
① Pancreatic duct-jejunal mucosa to mucosa anastomosis: As previously introduced, this method has fewer complications. Using a support tube and pulling it out of the body is the safest method.
② Pancreatic duct ligation: Proponents of this method believe it is simple, convenient, and time-saving, with fewer occurrences of pancreatic fistula disease. The method involves ligating the pancreatic duct with absorbable sutures and inserting the residual pancreas into the jejunal cavity for a double pancreaticojejunal anastomosis. The sutures are absorbed in about 2 weeks, by which time the anastomosis has healed. Limited case reports show no occurrence of pancreatic fistula disease, but it is unknown whether the pancreatic duct will narrow or obstruct.
③ Insertion of the pancreatic stump into the jejunum: This method involves thoroughly stopping bleeding from the residual pancreatic stump and directly inserting it end-to-end into the jejunum (if the jejunal diameter is small, a pancreaticojejunal end-to-side anastomosis can be performed). This method is simpler and less likely to result in pancreatic fistula disease. There is no definitive data on whether the pancreatic duct will narrow. However, some reports suggest that those who undergo this procedure do not need to rely on pancreatic exocrine supply.
④ Pancreatic duct occlusion (PDO): Pancreatic duct occlusion involves using an embolic agent to occlude the first to second level branches of the pancreatic duct, preventing the outflow of exocrine fluid and thus avoiding pancreatic fistula disease. Embolic agents include α-cyano-acrylate glue and neoprene latex 671. The domestically produced cyanoacrylate is currently more effective. We have used it for coronary vein (gastric coronary varices) embolization with ideal results and no observed toxic side effects. Carlo reported 51 cases of pancreaticoduodenectomy with pancreatic duct occlusion using neoprene latex, of which 2 cases developed pancreatic fistula disease. Ultrasound and CT scans showed no significant morphological changes in the residual pancreas. Ten cases followed up for 3 years showed no abnormal changes in pancreatic endocrine function, and none developed diabetes.
In pancreaticoduodenectomy, the management of the residual pancreas is crucial. Improper handling of the pancreatic duct can lead to pancreatic fistula disease, resulting in uncontrollable consequences. Our limited experience suggests that the first choice should be end-to-side anastomosis of the pancreatic duct mucosa to the jejunal mucosa at the pancreatic stump, with 4-6 sutures at the anastomosis. A minimally irritating silicone tube is inserted into the anastomosis and pulled out through the jejunum. This tube serves both as a support and allows observation of daily changes in pancreatic fluid volume, and is removed after 10-14 days. The jejunal seromuscular layer and pancreatic capsule are sutured properly, and external drainage is placed around the pancreaticojejunal anastomosis. Any small amount of pancreatic fistula disease can be fully drained externally without causing complications. In general, due to varying degrees of obstruction at the pancreatic head, the distal pancreatic duct is somewhat dilated, and most cases can undergo pancreatic duct-jejunal mucosa anastomosis. If the residual pancreatic duct is very thin and retracts into the pancreatic parenchyma, making mucosa-to-mucosa anastomosis impossible, a pancreatic stump jejunal invagination anastomosis is performed. Pancreatic duct ligation and occlusion are treatment methods that require further in-depth observation and exploration, and long-term practice through large case studies.
Through decades of clinical practice, there has been a further understanding of the indications and efficacy of the Whipple procedure. The Whipple procedure is most effective for ampullary cancer, with a resection rate as high as 90% and a 5-year survival rate of 40-45%. In contrast, the resection rate for pancreatic ductal adenocarcinoma is only about 10%, with a 5-year survival rate of 3.5-5%. The reasons for the poor efficacy of the Whipple procedure in pancreatic cancer, apart from the biological characteristics of pancreatic cancer itself, include some factors that the Whipple procedure cannot address, such as: infiltration of peripancreatic lymph nodes and blood vessels; about 6% of patients have multicentric pancreatic cancer; and about half of the patients have tumor presence at the pancreatic resection margin. In light of this, the surgical therapy for pancreatic cancer has been expanded, including total pancreatectomy and regional pancreatectomy. The pylorus-preserving pancreaticoduodenectomy for the treatment of pancreatic head cancer remains controversial.
Regarding the issue of resection margins in pancreatic head cancer. Wilett reported on 72 cases of pancreatic head cancer resection at Massachusetts General Hospital from 1978 to 1991. Pathological examination was performed on 67 specimens (93%), of which 37 (51%) had tumor invasion at the resection margins. There were 27 cases of invasion in the peripancreatic soft tissue, 14 cases at the pancreatic transection margin, and 4 cases at the common bile duct transection margin. No tumor invasion was found at the gastric or duodenal resection margins. In the entire group, 39 patients received postoperative radiotherapy and chemotherapy. Among the 35 patients with negative margins, the 5-year survival rate, local control rate, and distant metastasis-free rate were 22%, 43%, and 37%, respectively. Among the 37 patients with positive margins, the 3-year survival rate, local control rate, and distant metastasis-free rate were 6%, 22%, and 19%, respectively. Patients with positive margins did not survive beyond 41 months. Further analysis revealed that patients with negative margins had better pathological characteristics and histological types, or no vascular invasion, with 2-year and 5-year survival rates as high as 83%. Pathological examination found that 27 cases (38%) had tumor extension to the peripancreatic soft tissue margin, indicating that tumor invasion at the surgical margin or peripancreatic soft tissue margin accounted for a significant proportion.
(3) Total Pancreatectomy
In response to the issues with the Whipple procedure for treating pancreatic head cance




