| disease | Genital Tuberculosis |
| alias | Pelvic Tuberculosis |
Subcutaneous node disease remains one of the common diseases in our country. Subcutaneous nodes in female reproductive organs are not uncommon in pelvic inflammation, and the disease progresses slowly and covertly. The bacteria from subcutaneous nodes can be discharged with menstrual blood, posing a pestilential threat to the surrounding environment. To significantly reduce the epidemic of subcutaneous node disease in our country, attention should be paid to the prevention and treatment of subcutaneous nodes in reproductive organs.
bubble_chart Pathogenesis
1. Pathogenic Bacteria: Subcutaneous node bacilli are a type of slender bacilli with a tendency for branching growth, belonging to the genus Mycobacterium. They require prolonged staining time to be colored, but once stained, they can resist decolorization by acid-alcohol, hence they are also known as acid-fast bacilli. There are many species in this genus, with the human and bovine types generally being pathogenic to humans. The former primarily infects the lungs, while the latter first infects the digestive tract, then spreads to other organs, including reproductive organs, through various routes. In recent years, many countries have paid attention to infections caused by atypical mycobacteria in humans, including subcutaneous node-like lesions caused by subcutaneous node bacilli. The incidence of atypical mycobacterial lung infections in our country accounts for about 4.3% of mycobacterial lung infections.
Mitchison (1980) classified the subcutaneous node bacilli in subcutaneous node lesions into four categories based on their metabolic growth characteristics: Group A: Actively growing subcutaneous node bacilli, abundant extracellularly in early active lesions; Group B: As the disease progresses, they grow in acidic environments within macrophages, in smaller quantities; Group C: In neutral caseous lesions, they reproduce slowly or intermittently, in small quantities; Group D: Dormant, completely non-reproducing. These four groups of subcutaneous node bacilli show different responses to anti-subcutaneous node drugs, such as Group D, which is unaffected by any anti-subcutaneous node drugs and can only be cleared by the body's immune function or bacterial self-death.
The nature of disease caused by subcutaneous node bacilli is aerobic, but they can survive for a long time without oxygen, although they cannot reproduce. They have high nutritional requirements and grow slowly under good conditions, taking about 18 to 24 hours to reproduce a generation (compared to an average of 20 minutes for general bacteria), making cultivation difficult and usually requiring animal inoculation. However, clinical use of this slow growth characteristic has led to intermittent dosing regimens that achieve the same effect as continuous dosing.Subcutaneous node bacilli often undergo spontaneous genetic mutations, leading to primary resistance to certain anti-subcutaneous node drugs, with very few strains being primarily resistant to two drugs. Single-drug use easily eliminates sensitive strains and allows resistant strains to dominate, while using three drugs together almost eliminates resistant strains.
2. Transmission Routes: Infections are mainly secondary, primarily originating from lung and peritoneal subcutaneous node. Possible transmission routes include:
(1) Hematogenous spread: The main route of transmission. Subcutaneous node bacilli first invade the respiratory tract. Animal experiments have shown that injecting 2 to 6 subcutaneous node bacilli can cause lesions and rapid spread, forming lesions in the lungs, pleura, or nearby lymph nodes, then spreading to internal reproductive organs through the bloodstream, first the fallopian tubes, gradually affecting the endometrium and ovaries. Infections of the cervix, vagina, and vulva are rare.
Evidence suggests that if primary lung infection occurs close to menarche, the likelihood of hematogenous spread (i.e., pre-sensitization bacteremia) affecting the reproductive tract greatly increases, with no obvious tissue reaction or clinical symptoms at this time. Circulating subcutaneous node bacilli can be cleared by the reticuloendothelial system, but latent metastatic foci can form in the fallopian tubes, remaining dormant for 1 to 10 years or longer, until local immunity decreases and the latent foci reactivate, causing recurrent infection. This slow, asymptomatic process often results in complete absorption of the primary lung lesion without any radiologically diagnosable traces, which is almost a universal phenomenon at the time of definitive diagnosis of reproductive tract subcutaneous node.
(3) Lymphatic spread: Pathogens from intra-abdominal organ subcutaneous node lesions, such as intestinal subcutaneous nodes, spread retrograde through lymphatic vessels to internal reproductive organs. Since retrograde dissemination is required, it is rare.
(4) Primary infection: The possibility of direct infection of female reproductive organs forming a primary lesion is still debated. In male urogenital system subcutaneous nodes (such as epididymal subcutaneous nodes), patients can directly transmit the disease to their sexual partners through intercourse, forming primary vulvar or cervical subcutaneous nodes. Although such cases have been reported in the literature, subcutaneous node bacilli are not often found in semen, and it is impossible to rule out the existence of early asymptomatic primary sexually transmitted disease lesions in the lungs or other parts of the body in these cases. Sutherland (1982) found that among 128 female reproductive tract subcutaneous node patients, 5 cases (3.9%) had spouses with active urogenital tract subcutaneous nodes. However, among these 5 cases, 3 had spouses with subcutaneous nodes outside the reproductive tract.
When subcutaneous node bacilli infect a susceptible host, the local tissue first exhibits an inflammatory exudate of polymorphonuclear leukocytes, which is replaced by monocytes within 48 hours, becoming the initial site for the intracellular replication of subcutaneous node bacilli. When cellular immunity emerges, the subcutaneous node bacilli are eradicated, and the tissue undergoes caseous necrosis. If the infection site reactivates later, it leads to proliferative granulomatous lesions—subcutaneous node nodules. The typical histological image: central caseous necrotic tissue surrounded by concentric layers of epithelioid cells and multinucleated giant cells, with lymphocytes, monocytes, and fibroblasts infiltrating the periphery.
Among female reproductive organs, the fallopian tubes are the most frequently affected by subcutaneous nodes, accounting for 90-100%, mostly bilateral. The uterus is involved in 50-60%, almost entirely within the uterine membrane, rarely invading the muscle layer. Ovarian subcutaneous nodes often spread directly from infected fallopian tubes. Due to a tough white membrane surrounding the ovary, the infection rate is lower than in the uterine membrane, accounting for 20-30%, with at least half being bilateral. Cervical subcutaneous nodes originate from descending infections of uterine membrane subcutaneous nodes. Continuous cervical sections are not uncommon, accounting for 5-15%. Vaginal and external yin constipation nodes are rare, about 1%.
I. Fallopian Tube Subcutaneous Nodes Due to different infection routes, the initial stage of subcutaneous node salpingitis can be roughly divided into three types.
(1) Subcutaneous Node Perisalpingitis: The serosal surface of the fallopian tube is covered with grayish-white millet-sized nodules, initially not affecting the deep muscle and mucosal tissues, often part of diffuse subcutaneous node peritonitis or pelvic peritonitis. The entire pelvic organs, intestines, mesentery, and peritoneal serosa, as well as the uterine surface, are scattered with numerous grayish-white, varying-sized caseous nodules, ranging from a few mm to 1 cm in diameter. The entire serosal surface is congested and swollen, possibly with a small amount of ascites.
(2) Interstitial Subcutaneous Node Salpingitis: Initially, scattered small nodules appear in the submucosal or muscle layer, with lesions starting relatively localized, then progressing to invade the mucosa and serosa. This type is clearly hematogenously disseminated.
(3) Subcutaneous Node Endosalpingitis: The fallopian tube mucosa is first affected, often occurring at the distal end of the tube. The fimbrial mucosa swells, the lumen gradually enlarges, and mucosal folds adhere due to necrosis and surface epithelial shedding. However, the fimbriae may not necessarily close, possibly everting and remaining open. This type is mostly hematogenously infected, with secondary subcutaneous node peritonitis (bacilli invading from the fimbriae) being less common. Statistics show that only 13.5% of subcutaneous node peritonitis patients have genital subcutaneous nodes, while 32.8% of genital subcutaneous node patients also have peritoneal subcutaneous nodes, indicating that with open fimbriae, bacilli can directly spread from the fallopian tube to the peritoneum, explaining why subcutaneous node peritonitis is more common in females than males.
Depending on bacterial virulence and host immunity, the disease progresses roughly into two types:
1. Proliferative Adhesive Type: More common, 80% fall into this category, with slow progression and vague clinical manifestations. The fallopian tube wall thickens, appearing enlarged and rigid. Although the ostium may remain open, stenosis or obstruction can occur anywhere in the lumen. Cross-sections may reveal caseous nodular lesions in the mucosa and muscle wall (Photo 1), with chronic cases possibly calcifying. Sometimes mucosal hyperplasia occurs, with proliferative mucosal folds resembling adenocarcinoma. When lesions extend to the serosal layer or the entire tube is destroyed, caseous exudate may form, later invaded by granulation tissue, causing the tube to tightly adhere to adjacent organs. Sometimes adhesions with intestines, mesentery, bladder, and rectum form an inseparable inflammatory mass; severe cases may prevent surgical access to the abdominal cavity. However, ascites is not significant, and if present, often forms encapsulated effusions. Due to dense adhesions, intestinal obstruction may complicate.
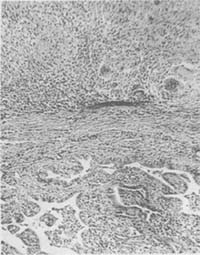
Photo 1 Fallopian tube subcutaneous node
2. Exudative type: It is an acute or subacute sexually transmitted disease process. The fallopian tube is significantly swollen, the membrane is severely damaged, the lumen is filled with caseous material, the wall is thickened, forming a subcutaneous node-type pyosalpinx. It is often tightly adhered to adjacent intestines, omentum, parietal peritoneum, ovaries, and uterus, but some may not adhere to surrounding tissues and are highly mobile, leading to misdiagnosis as ovarian cysts. There may be a few nodules on the surface of the serous membrane, which are generally not prominent and easily overlooked. Larger pyosalpinx often involves the ovaries, forming a subcutaneous node-type tubo-ovarian abscess. Sometimes, there may also be hematosalpinx or hydrosalpinx.
In the pus of subcutaneous node-type pyosalpinx, bacteria are usually no longer present, but secondary infections by common pyogenic bacteria are highly likely, which can cause severe lower abdominal pain, fever, leukocytosis, and other inflammatory symptoms. A rapidly enlarging painful mass can be palpated on one side. Such abscesses are prone to rupture into adjacent tissues, forming chronic fistulas. Incorrectly performing incision and drainage during the acute phase increases the risk of fistula formation and even intestinal obstruction.
II. Uterus subcutaneous node The size and shape of the uterus may appear normal. Subcutaneous node lesions are mostly confined to the endometrium, mainly at the fundus and cornua of the uterus, mostly descending and expanding from the fallopian tube lumen. In severe cases, the myometrium may be involved. Early changes in the endometrium are difficult to distinguish from endometritis. Sometimes, apart from a few scattered nodules, the rest of the endometrium and glands are essentially normal. The glucose content in the endometrium around the nodules is low, remaining in a proliferative state, while the endometrium further out shows typical secretory changes, so menstruation is usually unaffected. Due to the periodic shedding of the endometrium, there is not enough time to form extensive and severe endometrial subcutaneous node lesions. Caseation, fibrosis, and calcification are also rare. In severe cases, the myometrium may be involved, with partial or complete destruction of the endometrium, replaced by caseous tissue or forming ulcers, eventually leading to pyometra, complete loss of endometrial function, and amenorrhea. There is also a rare proliferative type of endometrial subcutaneous node, where the uterine cavity is filled with caseous granulomatous tissue, discharging large amounts of serous, foul-smelling leucorrhea, and the uterus becomes globularly enlarged, easily confused with uterine body cancer.
III. Ovarian subcutaneous node Often bilateral, with two types: perioophoritis and oophoritis. The former is caused by direct spread from fallopian tube subcutaneous nodes, with subcutaneous node granulomatous tissue on the ovarian surface, adhering to the fallopian tube to form a tubo-ovarian mass, often also adhering to the intestines or omentum. Oophoritis is caused by hematogenous spread, with lesions in the deep stroma of the ovary, forming nodules or caseous abscesses, while the cortex is often normal. This type is less common.
IV. Peritoneal subcutaneous node Diffuse miliary-type peritoneal subcutaneous nodes can appear as numerous scattered gray-white, varying-sized caseous nodules on the entire parietal peritoneum and serosal layers of abdominal and pelvic organs, with the entire peritoneal surface congested and swollen. In acute miliary subcutaneous nodes, ascites may occur. The nodules gradually fibrose, and the ascites is gradually absorbed, with subcutaneous node lesions temporarily improving or forming adhesions, leading to encapsulated effusions. Sometimes, caseous nodules may ulcerate, necrotize, or become infected with pyogenic bacteria, causing recurrent inflammation in the pelvic and abdominal cavities, eventually forming extensive adhesions and irregular masses, even leading to a "frozen pelvis."
V. Cervical subcutaneous node Cervical subcutaneous nodes are less common than the aforementioned lesions. The lesions can be divided into four types and are easily confused with cervicitis or cervical cancer, requiring biopsy and pathological examination for differentiation.
(1) Ulcerative type: The ulcer is irregular, superficial, with hard edges, clear boundaries, and an uneven base, appearing gray-yellow. This is the most common type of cervical subcutaneous node.
(2) Papillary type: Rare, appearing as papillary or nodular, gray-red, fragile, resembling cauliflower, similar to cauliflower-type cervical cancer.
(3) Interstitial type: This is a type of foxtail millet-sized lesion that spreads through the bloodstream, affecting all the fibromuscular tissues of the cervix, causing the cervix to swell and hypertrophy. It is the rarest form.
(4) Cervical mucous membrane type: Limited to the cervical canal, the mucous membrane directly spreads from the subcutaneous nodules of the uterine lining. Mucosal hyperplasia can be observed, with superficial ulcers and caseous nodules on the surface, and obvious bleeding on touch. Sometimes it can block the cervical canal, causing pyometra.
6. Vulvar and vaginal subcutaneous nodules: Both are relatively rare, mostly secondary infection sites caused by lesions of the internal genital tract subcutaneous nodules. The lesion may initially form small nodules on the labia or vestibular mucous membrane, which soon ulcerate, presenting as irregularly shaped superficial ulcers with an irregular base. The course is slow and difficult to cure. It may involve deeper tissues, forming sinuses with discharge of caseous material or pus. Vaginal subcutaneous nodule lesions closely resemble cancerous changes, and a biopsy is necessary for a definitive diagnosis.
bubble_chart Clinical Manifestations
Female reproductive organ subcutaneous node, due to its slow progression and relatively concealed pathological changes, exhibits significant variations in clinical manifestations depending on the severity and duration of the disease. Some cases may present no symptoms or signs other than infertility, while more severe cases may display typical subcutaneous node changes in the reproductive organs along with pronounced systemic symptoms.
1. Infertility Infertility is the primary symptom of genital subcutaneous node, with infertility being the sole complaint leading to medical consultation and diagnosis in 40-50% of patients with reproductive tract subcutaneous node. Statistics show that almost all patients with this disease have primary or secondary infertility, especially the former, which can account for up to 85%. Since the fallopian tubes are the first to be affected, the lesions often lead to obstruction or narrowing of the fimbrial end or other segments, or due to interstitial inflammation, causing abnormal peristalsis of the fallopian tubes or destruction of the mucosal cilia, affecting the transport of sperm or fertilized eggs and leading to infertility. Uterine membrane subcutaneous node hinders the implantation of fertilized eggs, resulting in infertility or late abortion.
2. Lower abdominal pain This is the second most common complaint among patients, accounting for about 25-50%. It generally presents as long-term dull pain in the lower abdomen, worsening before menstruation. If secondary suppurative bacterial infection occurs, there may be significant abdominal pain, fever, and tender masses, resembling acute pelvic inflammatory disease.
3. Irregular uterine bleeding Generally, menstruation is not affected, but when it causes pelvic organ static blood or inflammatory changes in the uterine membrane, various menstrual changes can occur.
4. Increased leucorrhea Increased leucorrhea can occur due to pelvic or uterine membrane subcutaneous node lesions. Especially in cervical subcutaneous node, the secretions may be purulent or purulent-bloody, sometimes even with contact bleeding or foul-smelling purulent-bloody discharge.
5. Coexistence with subcutaneous node in other organs Patients with genital subcutaneous node often have subcutaneous node in other organs. A detailed medical history and thorough physical examination (including silk examination) reveal that at least 80% have had subcutaneous node lesions outside the reproductive organs. About 10% coexist with active subcutaneous node lesions in other organs, most commonly pulmonary subcutaneous node, thoracic and peritoneal membrane subcutaneous node, followed by renal and bone subcutaneous node.
6. Systemic symptoms Patients with reproductive organ subcutaneous node may have common symptoms of subcutaneous node disease: fatigue, lack of strength, loss of appetite, weight loss, persistent low-grade fever in the evening, night sweating, and other chronic consumptive symptoms. However, most patients lack subjective symptoms and are often discovered during systematic physical examinations. The actual incidence of fever is twice as high as the subjective perception of fever, and it is more pronounced during menstruation.
7. Physical examination The results of physical examination vary greatly depending on the severity of the condition. Asymptomatic patients may show no abnormalities. If there are pelvic or peritoneal membrane subcutaneous node lesions, abdominal examination may reveal slight abdominal wall tension, tenderness, a doughy sensation, and signs of ascites.
The mobility of the uterus in patients with genital subcutaneous node may be normal or restricted due to adhesions. Generally, the uterus is smaller than normal. Typical fallopian tube subcutaneous node may present as bilateral hard cord-like structures. In severe cases, inflammatory masses of varying sizes may be palpable in the adnexa, fixed and tender. As the disease progresses, necrotic, caseous, and fibrotic tissues mix and accumulate, forming a large, hard, brittle, uneven, immobile mass filling the pelvis, even becoming board-like fixed, resembling advanced stage cancer lesions, but not adhering to the pelvic wall or sacrum, with the main ligaments not hardened and no hard nodules.
bubble_chart Auxiliary Examination
1. Laboratory Tests Routine laboratory tests are not particularly helpful in diagnosis. Most patients have normal total and differential white blood cell counts. The erythrocyte sedimentation rate (ESR) in chronic mild genital subcutaneous nodes is not as accelerated as in suppurative or gonococcal pelvic inflammation, but it often indicates that the lesion is still active, providing a reference for diagnosis and treatment. Therefore, ESR should be included as a routine test.
2. Chest X-ray Examination Since the majority of patients with this disease have secondary pulmonary infections, chest X-ray should be included as a routine examination. The focus should be on identifying any old subcutaneous node lesions or signs of pleural membrane subcutaneous nodes. Positive findings can provide some diagnostic reference for suspected cases, but a negative result should not be used to rule out the possibility of the disease.
3. Subcutaneous Node Tuberculin Test The standard technique involves intradermal injection of 0.1 ml of subcutaneous node tuberculin (purified protein derivative—PPD subcutaneous node tuberculin, equivalent to 5 subcutaneous node tuberculin units), with the size of the skin induration and erythema measured within 48 to 72 hours. A positive skin test indicates past infection but does not confirm active subcutaneous node lesions at the time of testing. Its value lies in raising the index of suspicion, especially for individuals with strong sexually transmitted diseases or adolescent girls, to determine if more specific tests are needed. It is important to note that a negative result does not completely rule out subcutaneous node disease, especially in cases of severe infection, corticosteroid use, elderly patients, or those with malnutrition.
4. Serological Diagnosis In recent years, enzyme-linked immunosorbent assays (ELISA) using purified protein antigens of subcutaneous node bacilli have been used to detect specific IgG and IgA antibodies against purified protein derivatives (PPD) in serum. This method has also been applied in clinical diagnosis of active subcutaneous node disease in China. Additionally, indirect immunofluorescence tests to detect specific antibodies in patient serum, combined with appropriate monoclonal antibody techniques, may enhance the sensitivity and specificity of subcutaneous node bacilli identification. The development and widespread application of these techniques provide rapid and sensitive diagnostic tools for genital subcutaneous nodes.
5. Special Examinations More than half of genital subcutaneous nodes involve the uterine endometrium, and endometrial tissue is relatively easy to obtain. Therefore, pathological examination of the endometrium, along with bacterial culture and animal inoculation of uterine cavity secretions, are definitive methods for diagnosing genital subcutaneous nodes. However, when subcutaneous node bacilli reach the uterine cavity from the fallopian tubes without causing significant endometrial lesions, pathological histological examination may not identify the infection, while bacterial culture or animal inoculation may yield positive results. Drug sensitivity tests can also provide information on the strain's medicinal properties, guiding clinical treatment. Thus, bacteriological examination is particularly important. However, the results of culture are influenced by factors such as the sensitivity of the culture medium, timing of sample collection, and the nature of the material. Additionally, the difficulty and time required for culture (6 to 8 weeks for results) limit the clinical utility of bacteriological examination. Currently, a combination of the above three methods is generally used, significantly improving diagnostic accuracy.
(1) Diagnostic Curettage: The optimal time for this procedure is 2 to 3 days before menstruation or within 12 hours of the onset of menstruation. Endometrial subcutaneous nodes often appear near the uterine cornua, so special attention should be paid to sampling from this area. Since early endometrial subcutaneous node lesions are small and scattered, the entire endometrium should be scraped to obtain sufficient material. Simultaneously, cervical endometrium and cervical biopsies should be taken and sent for separate testing to avoid overlooking cervical subcutaneous nodes. The endometrial specimens should be divided into two groups: one fixed in 10% formalin for pathological examination, and the other placed in a dry tube for immediate bacterial culture and animal inoculation. For pathological examination, serial sections are recommended to avoid misdiagnosis of fistula disease. In patients with prolonged amenorrhea, endometrial tissue may not be obtainable, but uterine cavity blood can be collected for bacterial culture and animal inoculation. Curettage may activate pelvic subcutaneous node lesions, so to prevent the spread of subcutaneous node disease, intramuscular streptomycin (1 g daily) should be administered starting 3 days before the procedure and continued for 4 days postoperatively.
A negative pathological examination result cannot yet rule out the possibility of a subcutaneous node. For clinically suspicious cases, a repeat diagnostic curettage should be performed at intervals of 2 to 3 months. If three consecutive examinations are negative, it can be considered that there is no intramembrane subcutaneous node in the uterus or that it has been cured.
(2) Bacterial culture and animal inoculation: Due to the low number of bacteria in the inner membrane subcutaneous node, direct smear staining microscopy of the inner membrane or uterine secretion has a low positive rate and no clinical practical value. Generally, half of the curettage specimen is taken for bacterial culture and animal inoculation. The uterine inner membrane fragments are finely ground in a sterile container and planted on an appropriate culture medium, checked once a week until 2 months or until a positive result appears. The ground inner membrane suspension is injected subcutaneously into the abdominal wall of guinea pigs, and after 6-8 weeks, the experimental animals are euthanized to take local lymph nodes, lumbar lymph nodes, and spleen for smear specimens, directly examined after staining, or further bacterial inoculation culture.
To avoid the risk of subcutaneous node dissemination caused by curettage, some advocate collecting menstrual blood for culture. The method involves placing a cervical cap on the patient's cervix during menstruation to collect menstrual blood for culture, or taking menstrual blood for culture under direct vision with a speculum on the first or second day of menstruation, but the positive rate is lower than that of uterine inner membrane bacteriological examination. The culture of cervical secretions during the menstrual intermission period, although not time-limited and can be repeated, has an even lower positive rate.
Although the above bacterial culture and animal inoculation can confirm the diagnosis, sometimes it is necessary to repeat the process to obtain a positive reaction of subcutaneous node bacteria, so it is generally determined that at least three negative results are needed to exclude subcutaneous node.
(3) Uterine tubal iodine contrast imaging: Uterine tubal imaging of genital subcutaneous node lesions can show certain characteristics, based on which, combined with high clinical suspicion of subcutaneous node, a diagnosis of genital subcutaneous node can be basically made.
There are two types of iodine contrast agents: iodine oil and water-soluble iodine. Since iodine water is less irritating than iodine oil, absorbs quickly, does not cause granuloma or oil embolism, and can show subtle tubal fistulas, iodine water is mostly used as a contrast agent, but its disadvantage is that if the film is taken deficiently, the iodine agent disappears in a short time.
The best time for imaging is within 2-3 days after menstruation. It is contraindicated for patients with inflammatory masses in the adnexa and fever. To prevent the activation and spread of the lesion, streptomycin can be injected intramuscularly for several days before and after the procedure.
Liu Boning et al. classified the characteristics of genital subcutaneous node on X-ray films of uterine tubal imaging into two categories based on their diagnostic value:
1. More reliable signs: Any clinical suspicion of subcutaneous node with any of the following characteristics can basically diagnose genital subcutaneous node.
(1) Multiple calcification points in the pelvic cavity: There are few conditions in the gynecological field that lead to pathological calcification in the pelvic cavity. Multiple calcification points in the area corresponding to the fallopian tubes, except for genital subcutaneous node, have very few other possibilities (Photo 2).
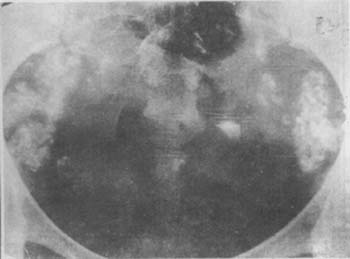
Photo 2 Pelvic silk film, showing multiple calcifications
(2) Mid-segment blockage of the fallopian tube, accompanied by iodine oil entering the interstitial ulcer or fistula formation of the fallopian tube causing perfusion defects.
(3) Multiple narrowings of the fallopian tube, appearing beaded.
(4) Grade III narrowing or deformity of the uterine cavity (Photo 3).
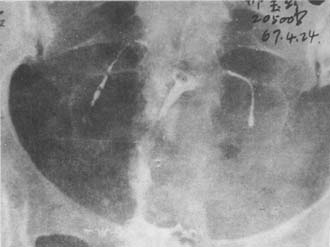
(1) Uterine cavity shrinkage, rough edges, fallopian tube blockage, rigidity, and beading

(2) Uterine contracture deformity, left fallopian tube slightly visible
Photo 3 Iodine oil imaging of uterine tubal subcutaneous node
(5) Iodine oil intracavitary perfusion (i.e., iodine oil enters the lymphatic vessels, blood vessels, or interstitial tissue. Accompanied by uterine cavity narrowing or deformation (Photo 4).

(1) Bilateral fallopian tube obstruction, with iodized oil entering the vessels of the isthmus, interstitial portion, and fundus of the fallopian tubes.
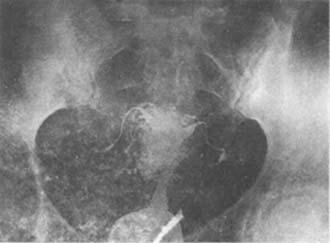
(2) Iodized oil enters the vessels and lymphatic vessels around the uterus.
Image 4: Iodized oil imaging of the uterus, fallopian tubes, and subcutaneous nodes.
(6) Ovarian calcification: Calcification signs appear in the area corresponding to the ovaries (refer to previous Image 2).
2. Possible signs: Clinically suspected subcutaneous nodes with any two or more of the following signs can be diagnosed as genital subcutaneous nodes.
(1) Isolated calcification points are shown in the pelvic plain film.
(2) The fallopian tubes are stiff and straight, with distal obstruction.
(3) The fallopian tubes are irregular in shape and obstructed.
(4) One side of the fallopian tube is not visualized, and the mid-segment is obstructed with interstitial iodized oil perfusion.
(5) The distal end of the fallopian tube is occluded, with perfusion defects in the lumen.
(6) Bilateral isthmic obstruction of the fallopian tubes.
(7) The edge of the uterine cavity is irregular and serrated.
(8) Iodized oil perfusion in the uterine interstitium, lymphatic vessels, or veins.
(4) Laparoscopy: Direct observation of the lesions is possible, and biopsies can be taken for pathological examination under the scope. Ascites can be directly smeared, acid-fast stained, examined under the microscope, or sent for bacterial culture, significantly increasing sensitivity. It is particularly valuable for differentiating uterine membrane ectopic disease or ovarian cancer. Many difficult cases that cannot be diagnosed by B-ultrasound and CT scans are confirmed by laparoscopy. However, for severe cases, dense adhesions often risk injuring the intestines and are contraindicated. In such cases, a small incision to take a sample is safer.
To further improve the diagnosis rate, it is essential not to overlook any suspicious signs. For instance, in infertility patients with scanty menstruation or amenorrhea, unmarried individuals with low-grade fever and emaciation, those with chronic pelvic inflammation that is difficult to cure, and those with a history of contact with subcutaneous node disease or a personal history of subcutaneous node disease, the possibility of genital subcutaneous node should be primarily considered.
Approximately 20% of genital subcutaneous node patients have a family history of subcutaneous node disease; more than 50% have had subcutaneous node disease outside the pelvis in the early stages, commonly pulmonary subcutaneous node, pleuritis, followed by subcutaneous node peritonitis, erythema nodosum, and renal and bone subcutaneous node, etc. If such a medical history is found, special vigilance for the possibility of this disease is required. Infertility is often the main or only symptom of this disease. Therefore, for such patients, a detailed inquiry about the history of subcutaneous node disease and a chest X-ray examination should be conducted. If genital subcutaneous node is suspected but lacks clear signs, further auxiliary diagnostic methods such as endometrial pathological examination, bacteriological examination, or hysterosalpingography should be used to confirm the diagnosis.
Some genital subcutaneous node patients have a long history of chronic consumption, poor appetite, emaciation, easy fatigue and lack of strength, persistent afternoon low-grade fever or menstrual fever, irregular menstruation, and long-term dull pain in the lower abdomen. In young girls, the presence of an inflammatory mass in the adnexa almost certainly diagnoses adnexal subcutaneous node. For adnexal inflammatory masses with no obvious history of infection, a slow course of disease, and poor response to general treatment, the possibility of subcutaneous node should be considered.
bubble_chart Treatment Measures
Once the diagnosis of genital subcutaneous node is confirmed, regardless of the severity of the condition, active treatment should be administered. This is especially true for patients with mild symptoms, as it is difficult to ascertain whether the lesion has become static or cured. To prevent the possibility of disease progression in the event of a future decline in immune function, even in the absence of obvious symptoms, the potential risks should be clearly communicated to persuade the patient to accept treatment.
Current treatments for genital subcutaneous node include general treatment, anti-subcutaneous node drug therapy, and surgical treatment.
I. General Treatment: Genital subcutaneous node, like subcutaneous nodes in other organs, is a chronic consumptive disease. The strength of the body's immune function plays a crucial role in controlling the progression of the disease, promoting lesion healing, and preventing recurrence after drug treatment. Therefore, patients in the acute phase need to rest in bed for at least 3 months. After the lesion is suppressed, they can engage in grade I activities, but they should also pay attention to rest, increase nutrition and intake of vitamin-rich foods, ensure adequate sleep at night, and maintain a happy spirit. Special comfort and encouragement should be given to infertile women to alleviate their concerns, which is beneficial for the recovery of their overall health.
II. Anti-subcutaneous node Drug Treatment: The advent of anti-subcutaneous node drugs has brought about significant changes and leaps in the treatment of subcutaneous node disease. Other treatment measures have mostly been abandoned, and cases that previously required surgery have been replaced by safer, simpler, and more effective drug treatments. However, to achieve ideal therapeutic effects, the five principles of rational treatment must be implemented: early, combined, appropriate, sufficient, and regular use of sensitive drugs. Early subcutaneous node lesions are in the bacterial reproduction stage; the earlier and fresher the lesion, the better the blood supply, and the easier it is for drugs to penetrate. Active treatment can prevent delays and the formation of refractory chronic caseous lesions. Combined drug use can kill naturally resistant bacteria or prevent their reproduction, greatly reducing the chance of developing anti-medicinal property subcutaneous node bacteria. However, due to the long course of drug treatment, patients often find it difficult to adhere to the regimen, leading to premature discontinuation or irregular medication, resulting in treatment failure. Therefore, clinicians should pay more attention to the principles of regularity and sufficient course, monitor the patient's treatment situation, strengthen supervision of the patient, avoid mid-term discontinuation or arbitrary drug changes, and prevent incomplete treatment, which can lead to resistance, refractoriness, and other adverse outcomes.
Due to the relatively small number of genital subcutaneous node patients, it is difficult to conduct well-controlled clinical trials. Therefore, the treatment protocols adopted are based on the treatment experience of pulmonary subcutaneous node.
(1) Mechanism of Action of Anti-subcutaneous node Drugs: The goal of anti-subcutaneous node drug treatment is to rapidly and thoroughly kill a large number of actively reproducing subcutaneous node bacterial populations (A populations) within the lesion, as well as to eliminate slowly and intermittently reproducing B and C subcutaneous node bacterial populations, to reduce recurrence. Currently, the most commonly used anti-subcutaneous node drugs are five in number.
1. Isoniazid (I, isoniazid): It has inhibitory and bactericidal effects on subcutaneous node bacilli and is the most commonly used drug in various treatment regimens. Its characteristics include good efficacy, small dosage, and ease of oral administration. The daily dose is 300mg orally or intramuscularly; if administered twice a week, the daily dose is 15mg/kg body weight. The disadvantage is that it can cause peripheral neuritis, with precursor symptoms such as a crawling sensation and burning sensation in the feet. The onset is related to vitamin B6 deficiency (due to increased excretion of vitamin B6 caused by taking I), so it is advisable to supplement with vitamin B6 30mg/d during treatment. Additionally, I has hepatotoxic effects. Grade I liver function abnormalities (elevated serum transaminase) can occur in 10-20% of patients during treatment, but even with continued treatment, serum SGOT levels can return to normal. Occasionally, progressive liver damage may occur, with a rate of 2-3% in patients over 50 years old, and alcohol consumption increases the risk, but it is rare in patients under 20 years old. Therefore, patients taking the conventional dose of I should be informed about hepatitis-related symptoms and instructed to report them to a doctor immediately. During monthly visits, attention should be paid to inquiries and liver function generation and transformation tests. If SGOT activity increases more than 5 times the normal value, the drug should be discontinued immediately and replaced with other anti-subcutaneous node drugs. This can significantly reduce the incidence of severe liver damage. Drug allergic reactions, such as lupus-like syndrome (antinuclear antibodies are often positive), wind-dampness syndrome, and granulocytopenia, are rare. Once detected, the drug should be discontinued immediately and replaced with other medications.
2. Rifampicin (R.rifampicin): It is a semi-synthetic derivative of rifamycin, to which subcutaneous node bacilli are highly sensitive. It is the only drug that has bactericidal effects on all three groups of bacteria A, B, and C. Oral dose: 10mg/(kg·d) up to 600mg/d or twice a week. Generally, it has low toxicity, with the most common side effects being gastrointestinal reactions and general allergic reactions, such as fever, headache, musculoskeletal pain (collectively known as flu-like syndrome), rash, etc. Occasionally, thrombocytopenia may occur, so patients should be advised to watch for skin ecchymosis, purpura, or hematuria.
3. Streptomycin (S): It has a greater bactericidal effect on extracellular subcutaneous node bacteria (Group A) than on intracellular bacteria (Groups B and C). Dose 1g/d, if twice a week, the daily dose is 20-30mg/kg body weight, requiring intramuscular injection, which is inconvenient for clinical application. The main side effects are chronic damage to the auditory and vestibular balance organs, causing deafness, tinnitus, vertigo, and balance disorders. For this reason, about 10% of patients need to stop the medication. During treatment, when patients visit, attention should be paid to inquiring about hearing and vestibular function, and patients over 50 years old should have regular high-frequency hearing tests. In addition, nephrotoxic complications may occasionally occur.
4. Pyrazinamide (pyrazinamid, Z): It is a highly effective bactericidal agent for subcutaneous node bacilli, but only kills intracellular bacteria. Oral dose 20-40mg/kg, up to a daily dose of 2g; for twice-weekly treatment, the daily dose is 50-70mg/kg, with few side effects, most commonly hyperuricemia and hepatotoxicity.
5. Ethambutol (ethambutol:E): It has a similar strong inhibitory effect on both intracellular and extracellular subcutaneous node bacilli and is also a commonly used anti-subcutaneous node drug in clinical practice. The usual dose is 15-25mg/(kg·d), or 50mg/kg, twice a week. Optic neuritis may occasionally occur, but is rare at doses <25mg/kg and can recover after stopping the medication. Therefore, during medication, attention should be paid to the patient's vision, and for those on high doses, regular vision and green vision tests are necessary.
(2) The relationship between anti-subcutaneous node drugs and some characteristics of subcutaneous node bacilli: As mentioned above, there are four types of subcutaneous node bacteria in subcutaneous node lesions. Anti-subcutaneous node drugs have different bactericidal and bacteriostatic effects on different types of bacteria and the pH of the bacterial environment. For example, I has a bactericidal effect on extracellular and actively growing bacteria in phagocytes (Groups A and B), S exerts its maximum effect on extracellular bacteria (Group A) only in an alkaline microenvironment, while Z is effective against intracellular Group B bacteria in an acidic environment. Therefore, as the disease progresses, the effects of the above anti-subcutaneous node drugs vary significantly.
In the early stages of subcutaneous node lesions, the local tissue pH is slightly acidic (pH 6.5-7), with Group A bacteria predominating, and I plays the main bactericidal role, followed by S. As the disease progresses, the tissue pH decreases, Groups B and C bacteria increase, Z and R play bactericidal roles, while I only has a bacteriostatic effect. After treatment, the inflammatory response is suppressed, the pH rises, and R becomes the main bactericidal drug, with S and I also having some effect, while Z's effect weakens. If inflammation recurs and the pH drops, returning to a state dominated by Group B bacteria, combined treatment with I and Z is superior to using I alone.
The selection of some treatment regimens and the duration of medication are designed and formulated based on the above rules.
(3) Common treatment regimens: In the past, considering that anti-subcutaneous node drugs only have an inhibitory effect on dormant subcutaneous node bacilli, treatment should continue until the host's immune system is sufficient to control residual infections. Additionally, more potent anti-subcutaneous node drugs such as Rifampin (R) and Ethambutol (E) were excluded from the standard treatment regimen and classified as second-line drugs, only considered when the standard regimen is ineffective or drug resistance has developed. To meet the above requirements, treatment generally needs to be maintained for 8 months. This prolonged treatment duration is referred to as long-term therapy, which patients often find difficult to adhere to, leading to treatment failure.
Over the past decade, through animal experiments and extensive clinical experience in treating subcutaneous nodes, treatments including I, R, E, or Z, as short as 9 months or even 6 months, have achieved results comparable to long-term therapies, with high cure rates and low recurrence, making them widely adopted. The only drawback of short-term therapy is the higher risk of liver perianal abscess. If treatment fails, R cannot be used as a backup drug.
Additionally, to ensure patients take their medication on time, it is recommended to administer the drug once in the morning on an empty stomach, which is easily accepted by patients and results in higher drug concentrations in the blood. The bactericidal effect of peak drug concentrations is better than that of consistently low blood concentrations; during the consolidation phase of the treatment, intermittent dosing is used, with effects similar to continuous dosing.
The current treatment regimen for subcutaneous nodes is often represented by symbols, such as 2IRSZ/4I3R3E3, indicating the first 2 months as the intensive phase, combining isoniazid (I), rifampicin (R), streptomycin (S), and pyrazinamide (Z); the following 4 months are the consolidation phase, with isoniazid, rifampicin, and ethambutol (E), administered three times a week.
1. Long-term therapy:
(1) Previous standard treatment regimen: includes S (daily dose 0.75~1g, intramuscular), I (daily dose 300mg), para-aminosalicylic acid (PAS) (daily dose 9~12g, divided into 2~3 doses), for 2~3 months; then I, PAS, for 10~15 months, with a total treatment duration of 12~18 months. This regimen is now largely obsolete.
(2) IRS (S daily dose 0.75g intramuscular, if intermittent dosing, 2~3 times a week, 1g each time; R daily dose 600mg administered at draught in the morning on an empty stomach; I regular dose), for 2~3 months, then I, R, total treatment duration 12 months.
(3) IRE (daily dose: I 300mg, R 600mg, E 750mg), for 2~3 months, then I, E, total treatment duration 12 months.
2. Short-term therapy:
(1) I (daily dose 300mg), R (daily dose 600mg), plus S (daily dose 1g, intramuscular) or Z (daily dose 1g), for 2 months; followed by I, R, for 4 months; if resistance to I is suspected, switch to E (daily dose 0.75~1g) from the start, strictly following the treatment requirements, which is an important measure to prevent the development of drug-resistant strains.
(2) 1 IRSZ/5S2I2Z2
(3) 2 IRSZ/4R2I2Z2
It is widely believed that the combination of R and I is more effective than any other treatment regimen, with a lower recurrence rate after discontinuation than any other drug treatment over the same period. However, continuous combined use of these two drugs for 18 months offers no additional benefits and carries the highest risk of liver toxicity. If R cannot be continued due to side effects, switch to the combination of ISE, discontinue S after 2 months, and continue the other two drugs for 16 months.
3. Application of Corticosteroids: Some suggest the use of corticosteroids as an adjunctive treatment to improve the inflammatory response caused by the disease. If chemotherapy is appropriate, it does not adversely affect the progression of the disease. Its indications include various types of subcutaneous nodular serositis, such as genital subcutaneous nodules complicated by subcutaneous nodular peritonitis, pelvic subcutaneous nodules with severe toxic symptoms. On the basis of effective anti-subcutaneous nodular drug combination therapy, prednisone (daily dose 30-40mg) is added, gradually decreasing after 1-2 weeks, with a treatment course of 4-8 weeks. For patients who are highly debilitated and have severe systemic symptoms, a smaller dose of prednisone (daily dose 30mg) can often promptly improve symptoms and reduce fever.
4. Application of Ofloxacin: Ofloxacin belongs to the quinolone class of antibacterial drugs. This class of drugs is entirely new, fully synthetic antibacterial agents with a broad antibacterial spectrum and potent antibacterial effects. They are well absorbed orally and have fewer toxic side effects, with gastrointestinal discomfort occurring in less than 1% of cases. Some patients may experience central nervous system reactions such as headache and insomnia. Long-term use is tolerated by patients without significant liver damage. According to Yew (1990), the combination of ofloxacin with second-line anti-subcutaneous node drugs, taken at a daily dose of 800mg for 8 to 12 months, can rapidly lyse bacteria and achieve satisfactory therapeutic effects. Currently, it has been used in patients with R or E resistant pulmonary subcutaneous node, where ofloxacin (300-600mg, taken once or twice daily on an empty stomach) is combined with two other anti-subcutaneous node drugs (PAC, kanamycin, Z, etc., at conventional doses and methods) to achieve good results, which is worth referencing.
III. Surgical Treatment: For genital subcutaneous node, anti-subcutaneous node drug treatment is the first choice, and surgical treatment is generally not considered. Surgery is only considered under the following conditions: ① Persistent pelvic mass after 6 months of drug treatment; ② Multiple drug resistance; ③ Persistent or recurrent symptoms (pelvic pain or abnormal uterine bleeding); ④ Recurrence of lesions after drug treatment; ⑤ Unhealed fistulas; ⑥ Suspected coexistence of reproductive tract tumors.
To avoid the spread of infection during surgery, reduce extensive pelvic organ adhesions and congestion that may complicate surgical procedures, and promote the healing of abdominal wall incisions, anti-subcutaneous node treatment should be administered for one to two months before surgery.
Although surgical complications are now rare, high vigilance is still required during surgery. Severe inflammatory adhesions may lead to injury to adjacent organs during separation, potentially causing fistulas. Therefore, blunt dissection should be avoided when separating adhesions. Once a separation line is established between organs, sharp dissection should be performed, with small cuts made progressively. Old intestinal adhesions do not need to be separated. It is safer to leave a small part of the uterine wall or fallopian tube attached to the intestine or bladder rather than forcibly removing all of it. If pelvic organ adhesions are severe and extensive, the round ligament should be identified, and the uterine fundus should be freed first to determine the surgical direction and proceed with the dissection.
If there are fistulas formed by pelvic subcutaneous node, preoperative X-ray examinations of the urinary system and the entire digestive tract should be conducted to fully understand the condition of the fistulas before surgery. Intestinal preparation with neomycin should begin several days before surgery.
If the uterus and bilateral adnexa are completely removed during surgery, all intraperitoneal lesions are cleared, and there are no coexisting subcutaneous node in other organs, then one to two months of postoperative anti-tuberculosis treatment is sufficient to prevent recurrence.
bubble_chart Follow-up Consultation
After treatment with anti-subcutaneous node medication, a close follow-up phase is required. Following combined, appropriate, regular, and complete treatment, recurrence or dissemination to other organs is extremely rare. Towards the end of the treatment course, it is advisable to repeat a chest X-ray, urine subcutaneous node bacterial culture, and diagnostic curettage. Repeat these examinations every 6 to 12 months within two to three years.
The following common gynecological diseases have signs that are extremely similar to those of subcutaneous nodules of the internal genitalia, and they often require differentiation in clinical practice.
1. Chronic nonspecific adnexitis and chronic pelvic inflammation
Patients often experience infertility, and the pelvic signs are very similar to those of subcutaneous nodules of the internal genitalia. However, the former often have a history of childbirth, late abortion, and acute pelvic inflammation; menstrual flow is generally heavier, and amenorrhea is rare. When chronic adnexitis does not respond to long-term treatment, hysterosalpingography or diagnostic curettage can be performed to rule out subcutaneous nodules of the genitalia.
2. Endometriosis
Ovarian endometriosis has many clinical manifestations similar to those of subcutaneous nodules of the genitalia, such as infertility, low-grade fever, abnormal menstruation, lower abdominal pain, and the formation of tender, fixed masses in the pelvis. However, patients with endometriosis often experience progressive dysmenorrhea, and 1-2 or more hard nodules can often be palpated in the rectouterine pouch, uterosacral ligaments, or posterior cervical wall. If the above two clinical manifestations are absent and diagnosis is difficult, laparoscopy can be performed to clarify the diagnosis.
3. Ovarian tumors {|105|} Subcutaneous nodular encapsulated effusion can sometimes be misdiagnosed as ovarian cysts or ovarian cystadenomas. Differentiation is relatively easy based on medical history, clinical symptoms, and signs such as the irregular surface, immobility, and fibrous adhesion thickening around the subcutaneous nodular adnexal mass. {|106|} Patients with advanced-stage ovarian cancer often present with cachexia, fever, and increased erythrocyte sedimentation rate. In addition to adnexal masses, metastatic lesions may appear at the base of the pelvis, making it difficult to differentiate from pelvic subcutaneous nodules combined with tubo-ovarian subcutaneous nodular masses. Clinically, ovarian cancer is often mistaken for subcutaneous nodules, leading to long-term anti-tuberculosis treatment, which delays the condition and endangers the patient's life. Conversely, pelvic subcutaneous nodules are sometimes misdiagnosed as advanced-stage ovarian cancer, leading to the abandonment of treatment. Fine-needle aspiration under B-ultrasound guidance can be performed to search for acid-fast bacilli and cancer cells. If the lesion is inaccessible, laparoscopy or exploratory laparotomy should be performed according to the situation to clarify the diagnosis early and obtain appropriate treatment to save the patient's life.





