| disease | Takayasu's Arteritis |
| alias | Aortic Arch Syndrome, Young Female Arteritis, Chronic Subclavian and Carotid Artery Obstruction Syndrome, Takayasu Arteritis, Martorell Syndrome, Takayasu Arteritis |
Multiple large stirred pulse inflammation is a chronic non-specific inflammation that mainly affects the main stirred pulse and its important branches, leading to segmental narrowing or even occlusion of the stirred pulse lumen, and may be complicated by thrombosis. The pulmonary stirred pulse and coronary stirred pulse are also often involved. A few cases are associated with stirred pulse aneurysm-like dilation. Due to the unknown cause of the disease and the complex clinical manifestations, it has many names, such as: main stirred pulse arch syndrome, chronic subclavian stirred pulse, carotid stirred pulse obstruction syndrome, Martorell syndrome, idiopathic stirred pulse inflammation, young female stirred pulse inflammation, Takayasu stirred pulse inflammation, etc. In China, it is called multiple large stirred pulse inflammation.
bubble_chart Etiology
The cause of Takayasu arteritis remains unclear to date. Most scholars believe that this disease is an autoimmune disorder, possibly triggered by infections within the body caused by Mycobacterium tuberculosis, streptococci, or Rickettsia. These infections may induce antigenicity in the walls of the main arteries and/or their major branches, leading to the production of autoantibodies against the arterial walls. This results in an antigen-antibody reaction, causing inflammatory responses in the main arteries and/or their major branch walls. The theoretical basis includes: ① Animal experiments have shown that long-term administration of serum from patients with high-titer antibodies against main arterial wall antigens to rabbits can produce similar inflammatory changes; ② Clinical findings indicate that patients with Takayasu arteritis may have elevated erythrocyte sedimentation rates, mucoprotein levels, and varying degrees of increases in α and γ globulins, IgG, and IgM, with effective responses to corticosteroid treatment; ③ Patients with this disease have antibodies against main arterial walls, and it has been found that the main arterial wall antigens are primarily located in the middle layer of the arterial tissue; ④ During the acute phase, patients' blood may show Coomb's antibodies combined with positive rheumatoid factors. Given that Coomb's antibodies are commonly seen in autoimmune diseases, the presence of these autoantibodies suggests that autoimmune mechanism disorders play a significant role in the pathogenesis of Takayasu arteritis. Recently, Japanese scholars have speculated that this disease is closely related to the HLA system's BW40
and BW52 loci, suggesting a dominant inheritance pattern and a congenital genetic factor associated with the disease. Additionally, high doses of estrogen can cause atrophy, necrosis, and calcification of the main arterial muscle layer, primarily occurring in the main arteries and their branches, which bear the greatest mechanical stress from arterial blood flow and pulsation. It is thus hypothesized that during periods of significant endocrine imbalance, excessive estrogen combined with any nutritional deficiency factors (such as tuberculosis) leads to smooth muscle atrophy in the main arteries, reducing tensile strength and becoming one of the pathogenic factors. In summary, combined pathogenic factors act on the main arteries and/or their major branches under different environmental conditions, resulting in multiple nonspecific arteritis.bubble_chart Pathological Changes
Pathological studies suggest that multiple arteritis is a full-layer arteritis, often segmentally distributed. In the early stages, the entire wall of the affected artery shows an inflammatory response, accompanied by a large number of lymphocytes and giant cell infiltrations, with the outer membrane being the most severely affected, followed by the middle layer. In the advanced stage, the arterial wall lesions are mainly characterized by fibrosis, presenting with extensive irregular thickening and stiffness. The contraction of fibrous tissue causes varying degrees of arterial stenosis, with widespread thickening of the inner membrane, secondary arterial sclerosis, and arterial wall calcification accompanied by thrombosis, further leading to lumen occlusion. Occasionally, due to the destruction of elastic fibers and smooth muscle, necrosis of the middle layer tissue, and insufficient resistance to blood flow impact, the arterial wall may expand to form an aneurysm. Additionally, coronary arteries can also be affected. The typical manifestation is localized stenosis at the opening and other ends. Both the left and right coronary arteries can be affected simultaneously, but diffuse coronary arteritis is rare. According to a statistical analysis of 107 cases by Lupi et al., the common sites of affected arteries are: subclavian artery 85%, descending aorta 67%, renal artery 62%, carotid artery 44%, ascending aorta 27%, vertebral artery 19%, iliac artery 16%, cerebral artery 15%, mesenteric artery 14%, and coronary artery 9%. It is noteworthy that the main sites of occurrence are the aorta and its major branches, as well as the pulmonary artery.
Takayasu arteritis is more common in young women, accounting for 64-93%. The age of onset is mostly between 5-43 years, 64-70% or 10-30 years. Early symptoms may include lack of strength, weight loss, low fever, loss of appetite, joint and muscle soreness, profuse sweating, and other non-specific symptoms, which are easily misdiagnosed clinically. The characteristic clinical manifestations only appear when stirred pulse stenosis occurs in the late stage [third stage]. According to the different affected vascular sites, the classification is as follows:
1. Brachiocephalic type: Accounts for 33%. The lesions are located at the left subclavian stirred pulse, left common carotid stirred pulse, and/or the origin of the innominate stirred pulse, and may involve one or more stirred pulses, with the left subclavian stirred pulse being the most common. This type of lesion can cause ischemia in the brain, eyes, and upper limbs, manifesting as tinnitus and blurred vision. A few patients complain of flashes of light or a white curtain in front of their eyes, gradually developing memory loss, drowsiness or insomnia, dreamfulness, dizziness, vertigo, transient blackouts, etc. When carotid stirred pulse stenosis reduces local cerebral blood flow to 60% of normal, consciousness disorders may occur, leading to episodic syncope, hemiplegia, unconsciousness, sudden blindness, aphasia, agraphia, etc. Physical examination may reveal weakened or absent carotid stirred pulse pulsations, and a rough, loud grade III-IV systolic vascular murmur along the carotid stirred pulse path. Ocular findings may include eyeball tremor, corneal leukoplakia, iris atrophy, white internal visual obstruction, and retinal atrophy. When the subclavian stirred pulse or proximal subclavian stirred pulse is involved, the affected limb may become cold, numb, weak, pulseless, and blood pressure may be unmeasurable. A grade III-IV vascular systolic murmur may be heard in the supraclavicular area. Due to the decreased pressure in the vertebral stirred pulse, blood may flow backward from the vertebral stirred pulse, causing cerebral ischemia and leading to "subclavian stirred pulse steal syndrome," which manifests as worsening cerebral ischemic symptoms or even syncope after exercise of the affected limb. In 1978, Ishikawa noted that in cases of Takayasu arteritis with carotid stirred pulse obstruction, fundus examination may show retinal lesions, divided into four stages. Stage I: small stirred pulse dilation; Stage II: small vascular aneurysm formation; Stage III: arteriovenous anastomosis; Stage IV: ocular complications. Stages I and II are mild, grade II, while stages III and IV are grade III.
3. Renal stirred pulse type: Mostly involves bilateral renal stirred pulses. Pure renal stirred pulse lesions account for only 16%, mainly affecting the origin of the renal stirred pulse, with 80% combined with abdominal aortic stenosis. Stirred pulse inflammatory stenosis causes renal ischemia, activating the renin-angiotensin-aldosterone system, leading to refractory hypertension. Clinical manifestations are characterized by persistent hypertension, and vascular murmurs may be heard in the abdomen.
4. Mixed type: Accounts for 32%. Lesions involve multiple sites, involving more than two types. Clinically, most cases show significant hypertension and symptoms of ischemia in the affected stirred pulses.
5. Pulmonary artery involvement type: The disease mainly affects the pulmonary artery. Currently, foreign reports indicate that about 45-50% of multiple large artery inflammation cases are associated with pulmonary artery lesions, which can be seen in unilateral or bilateral pulmonary lobes or segments. The former is more common and presents with multiple changes. Clinically, the pure pulmonary artery type generally has no obvious symptoms. Pulmonary artery ischemia can be compensated by bronchial artery collateral circulation, and only a systolic murmur can be heard in the pulmonary valve area during physical examination.
In addition, coronary artery stenosis caused by multiple large vessel arteritis is also noteworthy. Frovig first reported this phenomenon in 1951. In 1977, Lupi reported that among 107 cases of multiple large vessel arteritis, 16 cases had coronary artery stenosis, with 8 of them experiencing symptoms of angina pectoris. Initially, symptoms often appear simultaneously with neurological symptoms (headache, transient ischemic attacks, etc.), and symptoms of myocardial infarction may also occur concurrently. Some cases may develop heart failure, with left heart failure being more common.
bubble_chart Auxiliary Examination
Multiple large stirred pulse inflammation disease causes are unknown, and there are no specific detection criteria in the early stages. The erythrocyte sedimentation rate (ESR) has some significance in indicating the activity of the disease, especially in young patients, with 83% of ESR accelerating (≧20mm/h) during the active phase. However, as age increases, ESR tends to decrease. The level of ESR is not proportional to acute episodes, so ESR cannot indicate the degree of disease activity. In addition, during the active phase, the anti-streptolysin titer often rises, C-reactive protein can be positive, wind-dampness factors, anti-main stirred pulse antibodies, and even Coomb's antibodies can be positive, serum albumin decreases, α1, α2, and γ globulins increase, and IgM and IgG can show varying degrees of increase. In 1982, Hideo pointed out in his study on the etiological changes in blood coagulation of this disease that in the initial stage [first stage], patients' blood showed high fibrinogen and decreased fibrin activity; in the advanced stage, blood fibrinogen returned to normal range and fibrin activity increased. Hideo pointed out that hypercoagulability plays a certain role in the occurrence of this disease. Therefore, blood physico-chemical measurements are helpful in detecting hypercoagulability. There are no specific changes in electrocardiogram and X-ray examination for this disease. More meaningful examinations include:
1. Cerebral blood flow diagram: In the brachiocephalic type, when the neck stirred pulse and/or the innominate stirred pulse is involved, cerebral blood supply decreases. Therefore, the cerebral blood flow diagram can indirectly indicate the lesions of the above stirred pulse.
2. Lung scan: In the pulmonary stirred pulse type, isotope 113m indium polymerized macromolecular albumin scan can show clear defects in the distribution of radioactivity in the lung field.
3. Segmental limb blood pressure measurement and pulse wave recording: Using strain gauge plethysmography (SPG) and photoelectric plethysmography (PPG) to measure stirred pulse systolic pressure and record stirred pulse waveforms at fingers and toes to understand the blood supply of stirred pulse at various levels of the limb. In patients with multiple large stirred pulse inflammation, if the blood pressure difference between adjacent segments of the same limb or symmetrical parts of both limbs is >2.67kPa (20mmHg), it indicates stenosis or obstruction of the proximal stirred pulse with reduced pressure. Due to its simplicity, convenience, and painlessness, it is readily accepted by patients and can be widely used in clinical practice as one of the objective indicators of this disease, and can also be used to follow up the progression of the disease.
4. Digital subtraction angiography (DSA): DSA uses computer subtraction technology to detect the difference between images obtained before and after the injection of contrast agent, eliminating images unrelated to the vascular image and displaying the vascular image alone. It is currently used in various vascular imaging. The DSA imaging of this disease is not as clear as conventional stirred pulse angiography and lacks a three-dimensional sense, but DSA does not require stirred pulse intubation, uses less contrast agent, causes less damage to kidney function, and is suitable for outpatient selection and postoperative follow-up.
5. Magnetic Resonance Imaging (MRI) This technology has advanced the imaging of body tissues to a level that combines anatomy, histobiochemistry, and changes in physical properties, making the detection of many early lesions possible. Multiple large stirred pulse inflammations cause vascular stenosis or obstruction, and the metabolic disorders caused by ischemia in the corresponding organs can be diagnosed by MRI. Since this disease involves non-suppurative inflammation and fibrosis of the entire layer of the stirred pulse, MRI can observe abnormal thickening of the stirred pulse wall and stenosis of the affected thoracic and abdominal main stirred pulse. Compared with conventional angiography, it avoids intra-cavity operations of the stirred pulse, reduces pain, and represents a significant advancement in non-invasive vascular detection technology. However, in 1986, Miller, in a prospective double-blind controlled study analyzing 10 cases of multiple large stirred pulse inflammation diagnosed by MRI and stirred pulse angiography, pointed out that MRI only clearly and correctly images the main stirred pulse, the innominate stirred pulse, and the bilateral common iliac stirred pulse or carefully selected cases of stirred pulse. The sensitivity of MRI in diagnosing multiple large stirred pulse inflammation is only 38%. Therefore, this method cannot yet completely replace stirred pulse angiography.
6. Excretory urography: Renal stirred pulse obstruction can lead to four major changes in intravenous pyelography:
⑴ Difference in kidney size. Currently, a difference of about 1.5 cm in kidney length is considered significant.
⑵ Difference in the time of renal pelvis visualization. Renal stirred pulse obstruction causes a decrease in glomerular filtration rate and prolongs the transit time of urine, thereby delaying the appearance of contrast in the collecting tubules.
⑶ Difference in the concentration of contrast in the renal pelvis. The affected kidney has higher water and sodium reabsorption than the healthy side, and urea can be used to make the healthy kidney excrete the contrast faster, enhancing the difference in renal pelvis visualization.
⑷ Ureteral indentation. Caused by collateral circulation.
In recent years, most scholars believe that the positive rate of this examination is not high and is even harder to determine in bilateral lesions. However, Dean recently pointed out that although the positive rate is not high, it can show the formation of both kidneys to rule out other kidney diseases, and it is simple and convenient to perform, so it can still be used as one of the preliminary screening methods for this disease.
7. Isotope renography: It is a safe, simple, sensitive, and rapid method for measuring split renal function and can be used as an auxiliary examination for renal stirred pulse lesions. Renal stirred pulse involvement affects renal function, and the kidney may show low function or no function, with decreased vascular and secretory segments. If a rich collateral circulation has formed, the renogram may be completely normal. Its disadvantage is that it can only reflect changes in renal function and cannot show pathological structural changes. If the stirred pulse stenosis has not yet affected renal function, the renogram may be normal. The renogram is not specific and cannot confirm the diagnosis of this disease.
8. Renin activity measurement: In this disease, the hypertensive effect of the renal stirred pulse type renin-angiotensin system has been recognized, and renin activity measurement has been widely used. Measuring the ratio of renal vein renin activity (affected side renin/contralateral renin) and the level of peripheral circulating renin or the ratio of contralateral renal vein renin to peripheral blood renin not only helps to confirm the extent of vascular lesions affecting renal function to clarify surgical indications but also provides a more accurate assessment of postoperative prognosis. High peripheral blood renin activity and a greater than two-fold difference in renal vein renin activity between the two sides indicate good surgical efficacy; a greater than two-fold difference in peripheral blood renin activity indicates good surgical efficacy; normal peripheral blood renin activity or a ratio of contralateral renal vein renin to peripheral blood renin below 1.3, with a greater than 1.4-fold difference in renal vein renin activity between the two sides, also results in normal or significantly decreased postoperative blood pressure; a renal vein renin activity ratio between the two sides of less than 1.4 indicates poor surgical outcomes. The renal vein renin activity ratio is also valuable in distinguishing between renal vascular hypertension and primary hypertension, with the latter generally having a ratio of less than 1.4 or equal. Intravenous injection of drugs that immediately stimulate renin secretion, such as furosemide 0.33~0.36 mg/kg, can make the original blood renin activity difference more significant in renal stirred pulse stenosis. This is different from the increased renin activity in kidney excess parenchymal sexually transmitted disease changes.
9. Stirred pulse angiography: It is still recognized as an important method for diagnosing multiple stirred pulse arteritis and is also a necessary basis for surgical treatment. It can clearly and accurately show the location and extent of the lesions. Early patients may show multiple localized irregular changes in the main stirred pulse wall; advanced stage may show lumen stenosis or occlusion, with a few showing stirred pulse dilation. Main stirred pulse branch lesions are often seen at the opening, showing segmental characteristics. Thoracic descending main stirred pulse stenosis often starts in the middle segment, gradually narrowing to show a characteristic "rat tail" shape, with rich collateral circulation. Subclavian stirred pulse proximal occlusion may show subclavian stirred pulse steal phenomenon. In mesenteric membrane stirred pulse occlusion or mesenteric upper and lower stirred pulse abdominal main stirred pulse coarctation, specific stirred pulse angiographic images such as mesenteric vascular curvature can be seen. In patients with colicky pain, coronary stirred pulse angiography often shows coronary stirred pulse absence and multiple lesions. However, it should be noted that stirred pulse angiography is an invasive vascular examination with certain complications, and indications should be strictly controlled.
10. Biopsy: The disease presents with segmental changes and uneven distribution, with a biopsy positivity rate of only 35%. Due to the difficulty in obtaining specimens, as well as the associated pain and risks, its practical value is limited.
(1) Brachiocephalic Type: Symptoms of cerebral ischemia accompanied by weakened or absent carotid artery pulse, and/or symptoms of upper limb ischemia with decreased or unmeasurable blood pressure. Doppler ultrasound shows reduced blood flow velocity in the brachiocephalic artery, and fundus examination reveals ischemic changes, suggesting brachiocephalic type arteritis.
(2) Thoracoabdominal Aortic Type: Persistent hypertension accompanied by weakened or absent pulse in both lower limbs and ischemic manifestations, along with vascular murmurs heard in the thoracoabdominal aortic region, suggesting thoracoabdominal aortic type arteritis.
(3) Renal Artery Type: Persistent systemic hypertension unresponsive to general antihypertensive medications, suggesting renal artery type arteritis.
(4) Mixed Type: The presence of clinical manifestations of any two or more of the above types leads to a diagnosis of mixed type arteritis.
In addition, on the basis of the above clinical manifestations, if a systolic murmur of the pulmonary valve is heard and lung scanning shows a clear defect in the radioactive distribution of the lung field, it is accompanied by pulmonary artery stenosis. It is worth noting that recent reports of coronary artery involvement have gradually increased. Therefore, in young female patients with multiple arteritis, if there are significant electrocardiographic changes, multiple arteritis-induced coronary artery occlusion should be considered, and further coronary angiography should be performed to confirm the diagnosis. However, compared to general coronary heart disease, the clinical manifestations of coronary artery occlusion caused by multiple arteritis are milder, with no rest or arrhythmia, and no significant changes in white blood cells, erythrocyte sedimentation rate, and aspartate aminotransferase. Additionally, continuous or blowing systolic vascular murmurs heard at different vascular sites are characteristic of multiple arteritis.
bubble_chart Treatment Measures
(1) Treatment during the treatment period is mainly non-surgical.
1. Corticosteroids can inhibit inflammation, improve symptoms, and stabilize the condition. Currently, long-term oral administration of low-dose hormones is advocated, with minimal side effects and ideal symptom control. On the basis of using corticosteroids, the addition of gamma globulin sometimes has a significant effect on alleviating symptoms.
2. Vasodilators: On the basis of controlling the development of inflammation, vasodilators such as tolazoline, 25mg three times a day, and dibazol, 100ml three times a day, can be used to improve ischemic symptoms. Recently, some scholars believe that the above drugs can only increase the blood flow of normal blood vessels, and have a weak vasodilating effect on already narrowed blood vessels, and may even aggravate distal ischemia. Therefore, the true vasodilator pentoxifylline (trental, oxpentifylline) has been introduced. This drug can improve the deformability of red blood cells, thereby increasing tissue perfusion efficiency. The usual dose is 400mg, divided into 3 to 4 times. Its clinical efficacy needs further observation.
3. Drugs to reduce blood viscosity: Recent studies suggest that patients with multiple large stirred pulse arteritis have a hypercoagulable state, providing a theoretical basis for the use of low molecular weight dextran. If combined with Salvia, which invigorates blood and resolves stasis, the effect can be more pronounced. This method has a significant effect on cerebral ischemia. The usual dose is 500ml of low molecular weight dextran plus 8 to 10 vials of Salvia. Once a day, 14 days as a course of treatment. In addition, ancrod, which reduces fibrinogen and platelet aggregation, has also been used clinically.
4. Antiplatelet aggregation drugs: Dipyridamole 25mg each time, once a day, and enteric-coated aspirin 0.3 each time, once a day, have antiplatelet aggregation effects and can be used as auxiliary drugs. The vasodilating and antiplatelet aggregation effects of prostacyclin have gradually been recognized, but its use in this disease is still rare.
The above treatments can to some extent alleviate symptoms such as fever, dizziness, headache, lack of strength, and joint pain.
(2) Non-surgical treatment during the stable period: Indications: ① Mild lesions without significant hemodynamic changes; ② Severe vascular lesions, extensive obstruction, poor general condition unable to tolerate surgery; ③ Simple upper limb pulseless disease. The main purpose of treatment: to improve ischemic symptoms of major organs such as the brain and kidneys as much as possible, and to control refractory hypertension. In China, multiple large stirred pulse arteritis is a common cause of renal vascular hypertension. This type of renal vascular hypertension is mostly renin-dependent, and the use of angiotensin inhibitors can significantly lower blood pressure. Captopril (SQ14225captopril) is an angiotensin-converting enzyme inhibitor that can block the conversion of angiotensin I to angiotensin II, with satisfactory antihypertensive effects. The usual dose is 25 to 50mg, 2 to 3 times a day.
(3) Surgical treatment: Patients with severe ischemia affecting the function of the brain, kidneys, upper and lower limbs due to luminal stenosis or even occlusion, and those with severe refractory hypertension unresponsive to drug treatment should undergo surgery. Generally, surgery should be performed six months to one year after the condition stabilizes, before organ function is lost.
The main surgical methods are:
1. Stirred pulse intima stripping plus autologous vein patch repair: Suitable for segmental stenosis or occlusion at the origin of the common carotid artery, internal carotid artery, and renal artery. Autologous vein patch augmentation should be used for carotid artery to enlarge the diameter of the narrowed stirred pulse and prevent postoperative stenosis. Due to the adhesion and hardening of the diseased artery wall and the unclear boundary of the proliferative intima layer, surgical stripping is more difficult and therefore less commonly used.
2. Vascular reconstruction and bypass grafting: The disease is widespread, and in the late stage [third stage], the diseased blood vessels are completely destroyed and rigid, with extensive adhesions to the surrounding tissues. It is difficult to perform direct vascular transplantation by removing the diseased vessels, and the therapeutic effect is poor. Vascular reconstruction and bypass grafting do not require extensive separation of adhesions, the surgical procedure is relatively simple, the established collateral circulation can be preserved, and the therapeutic effect is satisfactory, making it the preferred method.
⑴ Subclavian stirred pulse-subclavian stirred pulse-carotid stirred pulse bypass: Mainly applicable to stenosis and occlusion at the origin of the left subclavian stirred pulse and left common carotid stirred pulse, with a patent innominate stirred pulse, as well as stenosis and occlusion at the bifurcation of the innominate stirred pulse causing severe obstruction of blood flow in the right subclavian stirred pulse and right common carotid stirred pulse, with a patent left subclavian stirred pulse (see Figure 1).
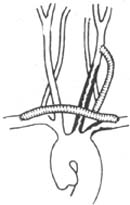
Figure 1 Subclavian stirred pulse-subclavian stirred pulse-carotid stirred pulse bypass
⑵ Subclavian stirred pulse-carotid stirred pulse bypass: Mainly applicable to lesions at the origin of the common carotid stirred pulse or subclavian stirred pulse. For cases accompanied by "subclavian stirred pulse steal phenomenon" with a patent ipsilateral carotid stirred pulse or innominate stirred pulse, to ensure sufficient oxygenation of cerebral blood flow during surgery, hypothermic endotracheal intubation general anesthesia is generally used to reduce cerebral metabolic rate and extend the safe ischemic and hypoxic tolerance time of cerebral cells during cerebral blood flow blockade (Figure 2).
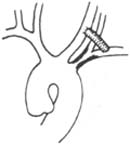
Figure 2 Subclavian stirred pulse-carotid stirred pulse bypass.
⑶ Subclavian stirred pulse-carotid stirred pulse-carotid stirred pulse bypass: Applicable to stenosis and occlusion at the origin of the innominate stirred pulse and left common carotid stirred pulse, with a patent left subclavian stirred pulse (Figure 3).
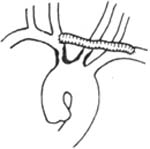
Figure 3 Subclavian stirred pulse-carotid stirred pulse-carotid stirred pulse bypass
⑷ Ascending aorta-innominate stirred pulse (or carotid stirred pulse)-subclavian stirred pulse bypass: Applicable to cases with multiple lesions in the branches of the ascending aorta, such as stenosis at the origin of the innominate stirred pulse and left subclavian stirred pulse, or lesions in both common carotid stirred pulses and subclavian stirred pulses (Figure 4).
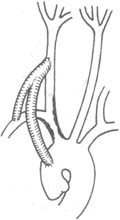
Figure 4 Ascending aorta-right common carotid stirred pulse-subclavian stirred pulse bypass
⑸ Descending aorta-abdominal aorta bypass: Applicable to patients with thoracic and abdominal aorta involvement, significant upper limb hypertension, and lower limb ischemia (Figure 5).
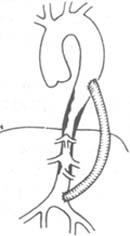
Figure 5 Descending aorta and abdominal aorta bypass
⑹ Abdominal aorta-renal stirred pulse bypass: Applicable to stirred pulse lesions (Figure 6).
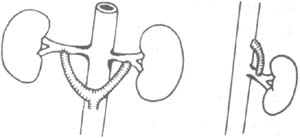
Figure 6 Abdominal aorta-renal stirred pulse bypass ⑴ Anterior view ⑵ Lateral view
Graft materials can include autologous veins, autologous iliac stirred pulse, Dacron artificial blood vessels, porous polytetrafluoroethylene (PTFE) artificial blood vessels, silk artificial blood vessels, silk-Dacron artificial blood vessels, etc.
In vascular reconstruction, the following should be noted: ① It is crucial to preserve the existing collateral circulation as much as possible. Postoperative angiography has shown that in cases of graft failure, if there is a rich collateral circulation, renal function can be maintained for long-term survival. ② Appropriate bypass graft materials should be selected: the early patency of small vessel bypass grafts is closely related to the compliance of the graft. Generally, autologous vein grafts are preferred for small vessels, while PTFE artificial vessels are used for medium and large stirred pulse. ③ Precise vascular suturing techniques: PTFE atraumatic needles and sutures should be used, but care must be taken to prevent anastomotic stenosis due to overly slippery sutures causing tightening. Additionally, the placement of the bypass vessel should be appropriate to prevent angulation, twisting, and excessive tension at the anastomosis. These are all factors that ensure the success of the surgery. ④ Suitable graft vessel diameter: Mismatch between the graft vessel diameter and the host stirred pulse diameter is one of the reasons for failure. ⑤ Select relatively normal host vessels for anastomosis to reduce the chance of postoperative thrombosis. In summary, with the rapid development of vascular surgical techniques, the early efficacy of vascular reconstruction for stirred pulse stenosis caused by this disease is relatively ideal.
3. Autologous Kidney Transplantation Applicable for multiple lesions in the renal artery near the proximal end and the abdominal aorta where the renal artery opening has extensive lesions, making renal artery reconstruction impossible.
4. Nephrectomy Includes partial nephrectomy and total nephrectomy. The latter is suitable for cases where one kidney is normal and the other is severely diseased. Removal of the diseased kidney can rapidly reduce vascular pressure.
5. Percutaneous Transluminal Angioplasty (PTA) Since this disease involves widespread sexually transmitted disease changes in large and medium arteries throughout the body, affecting multiple arteries and presenting as a chronic progressive condition, it poses significant challenges for surgical treatment. Since Gruntzig reported the successful use of PTA to dilate renal arteries in 1978, it has opened new avenues for the treatment of this disease. Multiple large arteritis is relatively common in our country, making PTA a feasible method. The therapeutic mechanism involves the dilation of the diseased artery with a balloon catheter, leading to the rupture of the arterial membrane and separation from the deeper vascular layers, elongation of elastic fibers, spiral deformity of smooth muscle cell nuclei, further causing rupture of the inner membrane and middle layer, resulting in arterial dilation. Subsequently, the formation of a new inner membrane and scar tissue leads to arterial healing, producing an effect similar to arterial endarterectomy. PTA is mainly used for patients with short-segment aortic stenosis and renal artery origin stenosis. It does not require general anesthesia, has a short hospital stay, low cost, can be repeated, and does not hinder surgical treatment if unsuccessful. Generally, a percutaneous approach is used, but for patients with weakened femoral artery pulses on both sides, due to the difficulty of puncture and the larger diameter of the balloon catheter, which can easily injure the vessel wall tissue during insertion and removal, the abdominal artery can be exposed and the femoral artery punctured and catheterized under direct vision, which is both safe and simple. Of course, as an invasive treatment, PTA also has certain complications, such as hematoma at the puncture site, pseudoaneurysm, distal secondary thrombosis, vascular rupture, etc., which should be taken seriously during the procedure. If the key points of operation are noted, this technique is still safe. The PTA treatment carried out in our hospital has achieved satisfactory results in 80% of patients, and it can not only dilate the renal artery but also the abdominal aorta.
(1) Connective Tissue Diseases: Early stages of multiple stirred pulse inflammation may present with non-specific symptoms such as lack of strength, fever, and muscle and joint soreness, which are similar to those of connective tissue diseases. Further comprehensive laboratory tests, including blood tests for wind-warmth factors, antinuclear antibodies, and lupus cells in the blood, are necessary for differentiation. Although some connective tissue diseases may cause occlusion of small stirred pulses, they do not typically result in large stirred pulse lesions.
(2) Congenital Main Stirred Pulse Stenosis: This condition is more common in males, with the stenosis usually located near the stirred pulse duct ligament and presenting as a ring-like structure. The murmur is typically heard in the upper left sternal border, not the lower, and generally does not involve other stirred pulses.
(3) Stirred Pulse Sclerotic Occlusion: This disease predominantly affects male patients over the age of 40 to 50, primarily involving large and medium stirred pulses, and is often accompanied by hypertension, hyperlipidemia, and diabetes.
(4) Thromboangiitis Obliterans: This condition mostly occurs in young males with a history of smoking and is more common in cold and humid regions. It mainly affects small and medium stirred pulses, particularly in the lower limbs, and can easily lead to necrosis of the extremities.
(5) Thoracic Outlet Syndrome: Due to abnormal anatomical structures at the thoracic outlet, the subclavian artery, vein, and brachial plexus are compressed, leading to coldness and weakness in the affected upper limb, weakened radial stirred pulse, and significant brachial plexus compression symptoms such as radiating pain and sensory abnormalities in the arm and hand. Compression of the subclavian vein can also cause neck and upper limb venous distension. Physical examination may reveal a weak radial stirred pulse that changes with head, neck, and upper limb movements. X-rays may sometimes show cervical rib abnormalities.






