| disease | Aortopulmonary Septal Defect |
| alias | Aorto-pulmonary Window |
Aortopulmonary septal defect, also known as aortopulmonary window, is a relatively rare congenital malformation of the great vessels. According to Stansel's 1977 statistics, fewer than 100 surgical cases had been reported in the literature at that time. The defect or window is located between the ascending aorta and the pulmonary trunk, with pathophysiology and clinical manifestations closely resembling those of patent ductus arteriosus. During the embryonic period (weeks 5 to 8), the aortopulmonary septum divides the truncus arteriosus into the ascending aorta and the pulmonary trunk. At the same time, the ventricular septum separates the ventricular cavity into the left and right ventricles. Eventually, the lower part of the aortopulmonary septum fuses with the upper part of the ventricular septum, ensuring that the left and right ventricles connect to the aorta and pulmonary artery, respectively. If this septation process is incomplete, depending on the location of the defect, it can result in an aortopulmonary septal defect, persistent truncus arteriosus, or a high ventricular septal defect.
bubble_chart Pathogenesis
Main-pulmonary stirred pulse septal defect leads to abnormal circulatory physiology. In the early stage, due to a large amount of blood flow shunting from the main stirred pulse to the pulmonary stirred pulse, the volume of blood returning from the pulmonary veins to the left heart chambers increases, placing a greater burden on the left ventricle. This results in left ventricular hypertrophy and strain, while the systemic circulation experiences relative blood flow insufficiency, leading to developmental delays or retardation. Due to pulmonary congestion, respiratory infections are more likely to occur. In the late stage (third stage), secondary sexually transmitted disease changes such as thickening of the pulmonary arteriole walls and narrowing of the lumen occur, increasing pulmonary stirred pulse resistance and pressure, overloading the right ventricle and causing combined hypertrophy of both left and right ventricles. When pulmonary stirred pulse pressure exceeds that of the main stirred pulse, a reverse (right-to-left) shunt forms, leading to systemic cyanosis.
bubble_chart Pathological ChangesA typical aortopulmonary septal defect is anatomically located just above the aortic valve, creating a communication between the aortic root and the pulmonary trunk (Figure 4②). The diameter of the defect can range from a few millimeters to several centimeters, generally exceeding 1 cm. In some patients, the defect is large, with its lower edge very close to the aortic valve, making it difficult to distinguish from a persistent truncus arteriosus based on appearance.
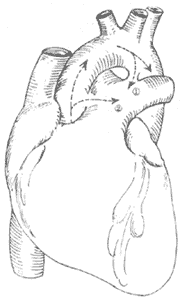
Figure 4 Schematic diagram of patent ductus arteriosus and aortopulmonary septal defect
① shows the ductus arteriosus between the descending aorta and the base of the left pulmonary artery; ② shows the aortopulmonary septal defect between the ascending aorta and the pulmonary trunk.
The clinical manifestations mainly depend on the amount of blood shunted from the main {|###|} artery to the pulmonary {|###|} artery, as well as whether secondary pulmonary {|###|} hypertension occurs and its severity. Due to the generally larger defect size compared to patent {|###|} ductus arteriosus and the proximity of the shunt to the heart, many patients die from congestive heart failure during infancy or early childhood. Survivors exhibit symptoms such as palpitations, shortness of breath, lack of strength, susceptibility to respiratory infections, and developmental delays, which are generally more pronounced than in patent {|###|} ductus arteriosus. In the advanced stage, when severe pulmonary {|###|} hypertension causes reverse shunting, systemic cyanosis occurs (unlike the lower-body cyanosis seen in patent {|###|} ductus arteriosus with pulmonary {|###|} hypertension). With the widespread use of antibiotics, {|###|} endocarditis has become rare.
Electrocardiography shows left ventricular hypertrophy or biventricular hypertrophy.
Chest X-ray reveals significant cardiac enlargement, prominence of the pulmonary {|###|} segment, and dilation of the ascending {|###|} aorta.
Ultrasound imaging demonstrates an abnormal communication between the ascending {|###|} aorta and the pulmonary {|###|} artery (Figure 1).
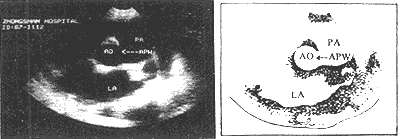
Figure 1: Ultrasound imaging showing aortopulmonary {|###|} septal defect (arrow).
AO = {|###|} aorta PA = pulmonary {|###|} artery LA = left atrium
Right heart catheterization reveals significantly higher oxygen content in the pulmonary {|###|} artery compared to the right ventricle. Right ventricular and pulmonary {|###|} artery pressures are usually elevated to some degree. If the catheter passes from the pulmonary {|###|} artery into the ascending {|###|} aorta, the diagnosis is confirmed.
bubble_chart Treatment Measures
The definitive diagnosis warrants surgical treatment. For patients with obvious pulmonary {|###|} artery hypertension but whose pulmonary {|###|} artery pressure remains lower than the main {|###|} artery pressure and whose left-to-right shunt murmur is still loud, early surgery should be pursued. If the pulmonary {|###|} artery pressure approaches or exceeds the main {|###|} artery pressure, the murmur is faint or absent, lip and finger cyanosis appears at rest or with grade I activity, {|###|} blood oxygen saturation is <90%, or total pulmonary resistance exceeds 10 Wood units, the window for surgery has been lost. At this stage, the defect has become a "safe" decompression channel for pulmonary {|###|} artery hypertension, and forcibly closing it surgically may promote right heart failure and accelerate disease progression.
The surgery employs a median sternotomy incision. After opening the pericardium to expose the heart and great vessels, the specific location and condition of the aortopulmonary {|###|} septal defect are identified. If the defect is high and tubular, two curved {|###|} clamps can be used to clamp the main {|###|} artery and pulmonary trunk walls on either side of the tube, followed by transection of the tube between the clamps (Figure 2). The cut ends are then closed with non-injury 3-0 synthetic fiber sutures in a continuous running fashion. In most patients, the defect is lower, with its inferior edge adjacent to the main {|###|} valve and the base of the coronary {|###|} artery, leaving almost no gap in between. If the defect is window-like, surgery must be performed under cardiopulmonary bypass (via femoral {|###|} cannulation). The main {|###|} artery distal to the defect is clamped, and the septal defect between the main {|###|} artery and pulmonary {|###|} artery is incised. The gap in the main {|###|} artery is sutured first. After de-airing the left ventricle and main {|###|} artery, the ascending main {|###|} artery clamp is released to restore coronary {|###|} perfusion before suturing the gap in the pulmonary {|###|} artery. Recently, some have adopted a simpler and more practical approach: under cardiopulmonary bypass and with the ascending main {|###|} artery clamped, the pulmonary trunk is incised, and the defect is sutured from within the pulmonary {|###|} artery lumen (Figure 3). For high-positioned defects, care must be taken to avoid injury to the right pulmonary {|###|} artery during surgery. For low-positioned defects, the coronary {|###|} artery must be protected. The difficulty and risks of the surgery are greater than those for patent {|###|} ductus arteriosus. According to Stansel's 1977 statistics, the mortality rate for surgeries performed without cardiopulmonary bypass was as high as 35%, while those with cardiopulmonary bypass had a mortality rate of 14%. Long-term outcomes depend on whether the patient had secondary pulmonary vascular disease before surgery and its severity.
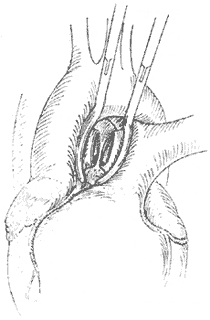
**Figure 2** The aortopulmonary {|###|} septal defect forms a tubular communication. Vascular clamps are applied to both ends, the connecting tube is transected, and the ends are sutured separately.
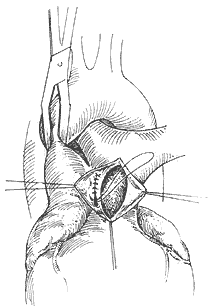
**Figure 3** Suturing the aortopulmonary {|###|} septal defect from within the pulmonary {|###|} artery lumen.





