| disease | Thoracic Aortic Dissection Aneurysm |
Blood flows through the ruptured inner membrane into the wall of the main stirred pulse, forming a hematoma within the wall of the main stirred pulse. As the hematoma expands, it separates the middle layer of the main stirred pulse wall into inner and outer layers, known as a main stirred pulse dissecting stirred pulse aneurysm. Sennertus in 1542 and Morgagni in 1761 described this condition. In 1826, Laennec referred to it as a dissecting stirred pulse aneurysm. The incidence of main stirred pulse dissecting stirred pulse aneurysm is approximately 5 to 10 cases per million people annually. The male-to-female ratio is about 3:1, and the majority of cases occur in individuals over the age of 40.
bubble_chart Pathological Changes
The main stirred pulse wall undergoes retrograde changes due to sexually transmitted disease, leading to a decrease in the adhesive force between various tissue layers. If the main stirred pulse wall is impacted by blood flow or the vascular nutrient vessels rupture, causing the inner membrane to break, then the middle layer of the main stirred pulse wall peels off, forming a wall hematoma with a thin outer layer and a thick inner layer. The stress generated by the heart's pulsation has the greatest impact on the ascending main stirred pulse and the proximal descending main stirred pulse, thus 60-70% of cases of dissecting stirred pulse aneurysms originate from the ascending main stirred pulse, and 25% originate from the proximal descending main stirred pulse. About 90% of cases are accompanied by hypertension. After the formation of a dissecting stirred pulse aneurysm, it can extend to the distal main stirred pulse, involving the entire length of the thoracic main stirred pulse and the abdominal main stirred pulse and its branches; extending to the proximal main stirred pulse involves the coronary stirred pulse and the main stirred pulse valve, leading to coronary circulation blockage or main stirred pulse valve insufficiency. If the dissecting lesion involves the common carotid stirred pulse, it causes cerebral ischemia symptoms; if the intercostal stirred pulse is involved, it can lead to spinal cord ischemia causing paraplegia; if the renal stirred pulse is involved, it leads to renal failure; if the iliac and femoral stirred pulses are involved, it can lead to limb necrosis. If the outer layer of the dissecting stirred pulse aneurysm ruptures into the pericardial cavity or the thoracic membrane cavity, it causes pericardial tamponade or massive hemothorax leading to death. In some patients, if the inner layer of the stirred pulse aneurysm ruptures into the main stirred pulse cavity, the main stirred pulse forms two blood flow channels, and the process of main stirred pulse wall dissection no longer progresses, and the condition is alleviated.
Classification: In 1965, DeBakey classified dissecting stirred pulse aneurysms into three types based on the location and extent of occurrence (Figure 4), which has been widely used clinically.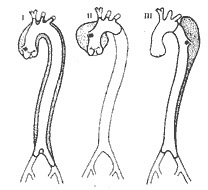
Figure 4 DeBakey classification of dissecting stirred pulse aneurysms
Type I: The inner membrane rupture is located in the ascending main stirred pulse, and the main stirred pulse wall dissection originates from the ascending main stirred pulse, involving the main stirred pulse arch, descending main stirred pulse, and may extend to the abdominal main stirred pulse.
Type II: The inner membrane rupture is located in the ascending main stirred pulse, and the main stirred pulse wall dissection is limited to the ascending main stirred pulse.
Type III: The inner membrane rupture is located in the proximal descending main stirred pulse distal to the left subclavian stirred pulse opening. The main stirred pulse wall dissects towards the descending main stirred pulse and may extend to the abdominal main stirred pulse, but does not involve the ascending main stirred pulse wall.
The Stanford classification divides into types A and B based on whether the ascending main stirred pulse is involved (Figure 5).
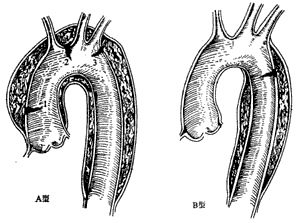
Figure 5 Stanford classification of dissecting stirred pulse aneurysms
Type A: The inner membrane rupture can be located in the ascending main stirred pulse, main stirred pulse arch, or proximal descending main stirred pulse. The extent of the dissecting stirred pulse aneurysm involves the ascending main stirred pulse, or even the main stirred pulse arch, descending main stirred pulse, and abdominal main stirred pulse. Stanford type A corresponds to DeBakey types I and II. Type A accounts for about 66% of cases.
bubble_chart Clinical Manifestations
Most patients with dissecting aortic aneurysm experience sudden, severe, knife-like or tearing pain in the abdomen, chest, or back. Chest pain may radiate to the neck and arms, resembling acute myocardial infarction. Even morphine-like drugs fail to alleviate the pain, which persists until the dissecting aneurysm ruptures, after which it subsides on its own. Patients often exhibit signs of shock such as pale skin, sweating, and peripheral cyanosis, but blood pressure remains higher than normal. Abdominal pain can be easily confused with acute abdominal conditions, but cases of dissecting aortic aneurysm rarely present with nausea, vomiting, abdominal tenderness, or muscle rigidity. If the dissection involves the ascending aorta, a diastolic heart murmur due to aortic valve insufficiency may be present. Involvement of the subclavian artery, common carotid artery, or iliac artery may result in local vascular murmurs, with weakened or absent pulses and blood pressure on the affected side. If the cerebral vessels are involved, the condition can be confused with hypertensive cerebral hemorrhage or cerebral thrombosis. Sudden paraplegia may occur if the intercostal arteries are affected.
bubble_chart Auxiliary Examination
Electrocardiogram (ECG) Examination: The ECG examination generally shows no abnormal signs, which can rule out the diagnosis of myocardial infarction. In cases with hypertension, left ventricular hypertrophy may be evident.
Two-dimensional echocardiography can show the ruptured flap of the inner membrane at the entry site of the dissecting aortic aneurysm.
bubble_chart Treatment Measures
The condition of aortic dissection is extremely critical, with a 24-hour survival rate of only 40%, a one-week survival rate of 25%, and a three-month survival rate of only 10%. The prognosis is even worse for patients with ascending aortic involvement, with a one-month survival rate of only 8%, while for those with only descending thoracic aortic involvement, the one-month survival rate can reach 75%. Hypertension accelerates the process of aortic wall dissection, exacerbates pain, and leads to early death due to aortic rupture causing hemopericardium, hemothorax, or mediastinal hematoma, adversely affecting the condition. Therefore, cases suspected of aortic dissection should be treated even before the diagnosis is confirmed by aortography. Medications should be administered to lower blood pressure, reduce peripheral vascular resistance, and decrease left ventricular contractility to prevent further expansion of the aortic wall dissection. The most commonly used drugs are Arfonad or sodium nitroprusside. Close monitoring of ECG, blood pressure, central venous pressure, pulmonary capillary wedge pressure, pulmonary artery pressure, and urine output is essential. Adjust the drug dose to maintain blood pressure between 13.3-16.0 kPa (100-120 mmHg) and ensure urine output is at least 30 ml per hour. Once the condition stabilizes, aortography should be performed immediately to determine the location and extent of the aortic wall dissection. In cases of aortic dissection, the aortic wall tissue is fragile and prone to rupture, making surgical procedures difficult and associated with high mortality. Cases with ascending aortic involvement, classified as Stanford type A or DeBakey type I and II, should undergo surgical treatment. Most cases classified as Stanford type B or DeBakey type III stabilize with medical treatment and can continue with it, but surgical treatment should be considered if the following conditions arise:
1. The aortic wall dissection continues to expand, with significant enlargement of the aortic wall hematoma, murmurs and weakened pulsations in the aortic arch branches or aortic valve, indicating involvement of the ascending aorta. Symptoms such as unconsciousness, apoplexy, limb pain and coldness, reduced or absent urine output suggest compression or obstruction of major aortic branches.
2. The aortic wall hematoma is at risk of imminent rupture, with signs such as a saccular aortic dissection or significant enlargement of the aortic dissection within hours, hemothorax or hemopericardium, and uncontrolled pain despite medical treatment.
3. Despite aggressive medical treatment for 4 hours, blood pressure remains uncontrolled and pain is not relieved.
Surgical procedure:
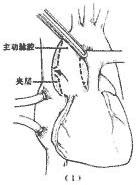
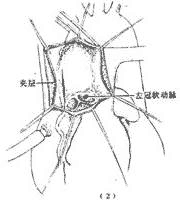
1. In cases where the aortic dissection lesion involves the ascending aorta, namely Stanford type A or DeBakey type I and II cases, a median sternotomy is performed, the pericardium is opened, and after systemic heparinization, a single venous drainage catheter is inserted into the right atrium. The arterial return cannula should be inserted into the femoral artery not involved by the aortic dissection lesion. Cardiopulmonary bypass is initiated, and the body temperature is lowered to around 25°C. Ice-cold saline is infused into the pericardial cavity for local profound cardiac cooling. A left atrial venting catheter is placed. The ascending aorta is clamped near the origin of the innominate artery. A longitudinal incision is made in the ascending aortic wall, opening the aortic lumen. Cold cardioplegic solution is infused through the left and right coronary ostia. The site of the intimal tear and whether the aortic dissection involves the aortic sinuses are inspected. If the dissection involves the aortic sinuses but the aortic valve function remains normal, the ascending aorta is transected above the sinuses. Then, small Dacron patches are placed inside and outside the aortic wall at the level of the aortic valve commissures, and mattress sutures are passed through the aortic wall to secure the commissures. Next, narrow circumferential strips of fabric are placed inside and outside the aortic wall at the proximal and distal aortic stumps to reinforce the aortic wall. The proximal and distal aortic stumps are then continuously sutured, followed by an end-to-end anastomosis of the ascending aorta or the interposition of a prosthetic graft between the two stumps (Figure 1). If the intimal tear involves the aortic arch, part of the aortic arch may be resected and replaced with a prosthetic graft, which is then wrapped with the aortic wall for reinforcement and hemostasis.
 |  |
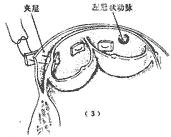 | 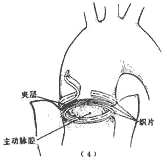 |
Figure 1 Surgical method for ascending aorta stirred pulse interlayer stirred pulse tumor
(1) Ascending aorta stirred pulse incision; (2) Exposure of the lesion; (3) Suture fixation of the junction; (4) End-to-end anastomosis of the ascending aorta stirred pulse
If the lesion requires resection of the aortic valve, after removing the aortic valve and the diseased segment of the ascending aorta, perform aortic valve replacement and artificial vascular implantation, or use a valved artificial vessel. The artificial valve membrane end is sutured to the aortic valve annulus. A small window is cut on the artificial vessel and anastomosed to the aortic wall near the coronary artery opening, or a saphenous vein bypass is performed between the artificial vessel and the coronary artery to ensure coronary blood flow. The other end of the artificial vessel is anastomosed end-to-end with the distal segment of the ascending aorta (Figure 2).
Figure 2 Ascending aorta stirred pulse interlayer stirred pulse tumor undergoing artificial valve membrane replacement and artificial blood vessel implantation
2. In cases where the ascending aorta stirred pulse wall dissection lesion only involves the descending aorta, i.e., Stanford type B or DeBakey type III cases, most patients stabilize after medical treatment and do not require surgical intervention. For those whose condition progresses and necessitates surgery, the affected segment of the thoracic descending aorta is excised and replaced with an artificial blood vessel. The length of the excised aortic segment is minimized according to the lesion's extent. To prevent spinal cord and visceral ischemic and hypoxic damage caused by blocking the descending aorta, surface hypothermia anesthesia combined with drug control of upper body blood pressure can be employed; temporary external shunt catheters are used; left heart bypass or femoral vein-femoral artery bypass is performed. After blocking the aorta proximal and distal to the lesion, cold lactated Ringer's solution is pressurized and injected to lower the spinal cord temperature, which also protects the spinal cord.
A left posterior thoracotomy incision is made, entering the chest through the 5th or 6th rib bed. An aortic clamp is placed between the proximal descending aorta or the common carotid artery and the left subclavian artery, and another clamp is placed distal to the lesion on the descending aorta. The affected segment of the descending aorta is longitudinally incised to observe the condition of the intercostal artery openings on the posterior aortic wall, preserving as much of the posterior aortic wall at the intercostal artery openings as possible. After excising the affected segment of the descending aorta, the proximal and distal aortic wall ends are reinforced with a circular strip of fabric, which is then continuously sutured to fix the aortic wall to the fabric. A segment of artificial blood vessel of appropriate length, diameter, and shape is then anastomosed end-to-end with the proximal and distal aortic ends (Figure 3). If part of the posterior aortic wall and intercostal arteries are preserved, one end of the artificial blood vessel needs to be trimmed obliquely. After completing the anastomosis, the distal aortic clamp is released first. If there is fistula disease bleeding at the anastomosis site, additional sutures are added, and then the proximal aortic clamp is slowly released and removed. The implanted artificial blood vessel is wrapped and sutured with the interlayer stirred pulse tumor wall for reinforcement and hemostasis.
⑴ Show the reinforcement of the proximal and distal segments of the main stirred pulse wall by a circular narrow strip of fabric, with the inner and outer cut ends
⑵ After completing the artificial blood vessel transplantation, use the layered stirred pulse aneurysm to wrap and suture, serving to reinforce and stop bleeding
Figure 3: Layered descending main stirred pulse aneurysm resection and artificial blood vessel transplantation
Postoperative management is the same as for general major cardiovascular surgeries, but close attention should be paid to blood pressure to prevent hypertension. Follow-up examinations should monitor whether the residual stirred pulse aneurysm false lumen is enlarging; if enlargement occurs, timely intervention is necessary to prevent rupture.
Surgical treatment outcomes: The surgical mortality rate for layered stirred pulse aneurysms remains high. For cases involving the ascending main stirred pulse, the surgical mortality rate is 20-40%, while for cases limited to the descending main stirred pulse, the rate is 25-60%. The main causes of death include rupture and bleeding of the main stirred pulse or anastomosis, acute heart failure, cerebrovascular disease, mesenteric or renal vascular infarction, and pulmonary complications. Approximately 10-20% of cases develop paraplegia postoperatively. The 5-year survival rate is about 50%, decreasing to 30% and 5% at 10 and 20 years postoperatively, respectively.






