| disease | Descending Thoracic Aortic Aneurysm |
The most common type of thoracic aortic aneurysm is the descending aortic aneurysm.
bubble_chart Pathological Changes
In the past, aortic aneurysms were mostly caused by syphilis, but currently, the majority are caused by atherosclerotic lesions. Factors such as advanced age and hypertension increase the incidence of atherosclerotic lesions. Other causes include trauma, bacterial infections, and medial necrosis of the aorta. Most descending aortic aneurysms occur in the proximal descending aorta, distal to the left subclavian artery. The affected aorta expands into a spindle shape, varying in length, and sometimes involving the entire descending aorta or even extending into the proximal abdominal aorta. The aneurysm grows slowly and eventually ruptures, leading to fatal hemorrhage. After diagnosis is confirmed by chest X-ray, the average survival period is about 3 years.
bubble_chart Clinical Manifestations
The descending thoracic aortic aneurysm may not present any symptoms in the early stages. As the aortic aneurysm enlarges, patients often complain of chest pain between the shoulders, sometimes with pain in the lower back, shoulders, upper limbs, or neck. The pain is often a persistent dull ache. Compression of the left main bronchus by the aortic aneurysm can lead to dyspnea, while rupture into the lung or bronchus may cause hemoptysis. Compression of the left recurrent laryngeal nerve can result in hoarseness.
Chest X-ray and computed tomography scans can reveal the shadow of the aortic aneurysm and may show pulsation or thrombosis. Aortic angiography can confirm the diagnosis and demonstrate the location, morphology, and extent of the aortic aneurysm.
bubble_chart Treatment MeasuresThe surgical treatment for descending aortic stirred pulse tumors involves resection of the stirred pulse tumor and replacement with an artificial blood vessel. During the procedure, the descending aortic stirred pulse needs to be clamped. To avoid resulting hypertension in the upper body and ischemic or hypoxic damage to the spinal cord and internal organs, a temporary external shunt catheter made of silicone with a diameter of 7–9 mm can be placed between the proximal and distal segments of the stirred pulse tumor. This allows partial blood flow from the left subclavian stirred pulse or aortic arch to the femoral stirred pulse or distal descending aortic stirred pulse. The external shunt catheter is removed after the artificial blood vessel replacement is completed. Another method is to perform left heart bypass, which can be achieved through: ① Left atrial-femoral stirred pulse bypass: After systemic heparinization, a drainage catheter is inserted into the left atrium, and a blood supply catheter is inserted into the femoral stirred pulse. Partially oxygenated blood from the left atrium is pumped into the femoral stirred pulse to supply the lower body, while the heart continues to supply the upper body. ② Femoral vein-femoral stirred pulse bypass: After systemic heparinization, a drainage catheter is inserted into the left femoral vein, and a blood supply catheter is inserted into the left femoral stirred pulse. Blood drawn from the femoral vein is oxygenated in an oxygenator and then pumped into the femoral stirred pulse. When using left heart bypass, the perfusion flow for the lower body should be maintained at around 1000 ml per minute, and a perfusion pressure above 4 kPa (30 mmHg) is sufficient to protect kidney function.
If the stirred pulse tumor lesion is relatively localized and the aortic stirred pulse clamping time is within 30 minutes, only surface cooling is needed to enhance the spinal cord's tolerance to ischemia and hypoxia. Intravenous infusion of sodium nitroprusside can be used to control upper body hypertension, eliminating the need for external shunts or left heart bypass. After entering the thoracic cavity, the proximal and distal segments of the stirred pulse tumor are dissected. In most cases, the proximal end of the stirred pulse tumor is below the left subclavian stirred pulse, requiring only a clamp on the distal aortic arch. If the proximal end of the stirred pulse tumor is close to the opening of the left subclavian stirred pulse, the aortic arch must be clamped between the left common carotid stirred pulse and the left subclavian stirred pulse, while also clamping the left subclavian stirred pulse. A clamp is then placed on the distal descending aortic stirred pulse. After clamping, the stirred pulse tumor is longitudinally incised. The openings of the intercostal stirred pulses on the posterior aortic wall are ligated. However, for long-segment descending aortic stirred pulse tumors, efforts should be made to preserve several intercostal stirred pulses. To achieve this, one end of the descending aortic stirred pulse can be obliquely transected, preserving the posterior aortic wall where the intercostal stirred pulses originate. A pre-clotted artificial blood vessel with a slightly smaller diameter than the aorta and appropriate length is then anastomosed end-to-end to the proximal and distal aortic segments without fistula disease bleeding. After completing the anastomosis, the distal aortic clamp is first released to expel any trapped air in the artificial blood vessel. Once the anastomosis is confirmed to be free of fistula disease bleeding, the proximal and distal aortic clamps are slowly removed. The stirred pulse tumor wall is then wrapped tightly around the artificial blood vessel, and the incision edges are sutured (Figure 1).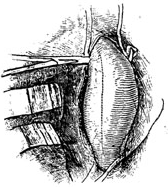
⑴ After clamping, the stirred pulse tumor is longitudinally incised.
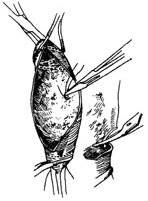
⑵ The openings of the intercostal stirred pulses on the posterior aortic wall are ligated.

⑶ The artificial blood vessel is sequentially sutured to both ends of the descending aortic stirred pulse.
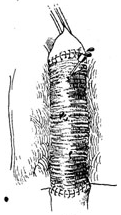
⑷ The distal aortic clamp is released to expel trapped air from the artificial blood vessel.
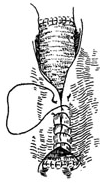
⑸ The proximal and distal aortic clamps are slowly removed, and the stirred pulse tumor wall is wrapped tightly around the artificial blood vessel, suturing the incision edges.
Figure 1 Descending aorta stirred pulse stirred pulse tumor resection and artificial blood vessel transplantation




