| disease | Amniotic Fluid Embolism |
| alias | AFE |
Amniotic fluid embolism (AFE) is a rare but dangerous obstetric complication. The reported incidence varies in the literature, ranging from 1:5,000 to 1:80,000, with a mortality rate as high as 50-86%. According to the National Maternal Mortality Research Collaborative Group, among the 7,485 maternal deaths recorded in 21 provinces, municipalities, and autonomous regions across China between 1984 and 1988, AFE accounted for 5.4% of total maternal deaths, ranking as the fourth leading cause of death.
bubble_chart Etiology
The solid components in amniotic fluid enter the maternal circulation, causing a series of pathophysiological changes. These solid components include squamous epithelium, lanugo, vernix caseosa, meconium, mucin, etc. The predisposing factors are as follows: ① Mostly seen in multiparous women; ② Often associated with premature rupture of membranes or artificial rupture of membranes; ③ Commonly caused by excessive uterine contractions or improper use of oxytocin; ④ Placental abruption, placenta previa, uterine rupture, or operative delivery increase the risk of amniotic fluid embolism; ⑤ Retention of a dead fetus can raise the incidence of amniotic fluid embolism. The conditions for amniotic fluid entering the maternal circulation are ruptured membranes, strong uterine contractions, and open blood vessels. The entry pathways include the endocervical veins and lower uterine segment veins, placental marginal venous sinuses, and injured uterine sinuses, such as uterine rupture or cervical lacerations.
1. Acute respiratory and circulatory failure: The amniotic fluid contains particulate matter from the fetus. Once it enters the maternal circulation, these particles cause mechanical obstruction of small vessels through embolism. These particles also possess chemical mediator properties, stimulating lung tissue to produce and release vasoactive substances such as prostaglandin F2α
, E2, and serotonin. This leads to pulmonary vasospasm, resulting in elevated pulmonary artery pressure, increased right heart load, and a sharp drop in left atrial pressure. Consequently, cardiac output significantly decreases, pulmonary return flow markedly declines, and the ventilation-perfusion ratio becomes imbalanced, ultimately leading to peripheral circulatory failure, acute right heart failure, and acute respiratory failure. 75% of fatal cases die from this cause. Additionally, antigenic substances in the amniotic fluid acting on the fetus can trigger allergic reactions leading to shock.2. Acute disseminated intravascular coagulation (DIC): The entry of amniotic fluid into the maternal circulation causes coagulation dysfunction. It is generally believed that the procoagulant substances in amniotic fluid resemble tissue thromboplastin (Factor III), activating the extrinsic coagulation pathway and leading to DIC. Furthermore, amniotic fluid also contains Factor X-activating substances, pulmonary surfactants, and trypsin-like substances from meconium. These procoagulants promote platelet aggregation and the conversion of prothrombin to thrombin, similarly activating blood coagulation via the extrinsic pathway, resulting in acute DIC. Fibrinogen levels in the blood are consumed and decrease, while the fibrinolytic system is activated, causing hyperfibrinolysis and coagulation disorders. Additionally, the accumulation of fibrin degradation products, combined with the amniotic fluid's inhibition of uterine contractions, reduces uterine tone, leading to non-coagulable uterine bleeding.
3. Multi-organ injury and acute respiratory and circulatory failure: Pathological changes such as DIC often involve multiple maternal organs, with shock kidney, acute tubular necrosis, extensive hemorrhagic liver necrosis, and pulmonary and splenic hemorrhage being the most common. Clinically, this manifests as acute liver and kidney failure. When two or more vital organs simultaneously or sequentially experience functional failure, it is termed multiple system organ failure (MSOF), with a mortality rate approaching 100%. The pathophysiological changes of amniotic fluid embolism are detailed in Figure 2.
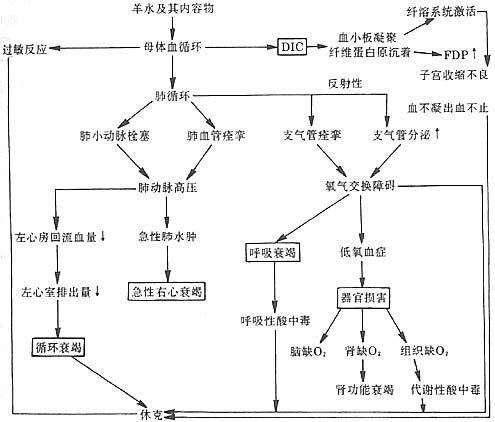
Figure 2 Schematic diagram of the pathophysiological changes in amniotic fluid embolism.
bubble_chart Clinical Manifestations
Amniotic fluid embolism has a rapid onset, and often by the time many laboratory tests are performed, the patient has already died. Therefore, to achieve early diagnosis, it is essential to be familiar with the predisposing factors and prodromal symptoms. In most cases, the initial symptoms often include shivering, dysphoria, coughing, shortness of breath, cyanosis, and vomiting. If the amount of amniotic fluid entering is minimal, the symptoms may be mild and sometimes resolve on their own. If the amniotic fluid is turbid or the volume entering is larger, typical clinical manifestations will follow.
1. Respiratory and circulatory failure: Based on the severity, it can be classified into fulminant and slow-onset types. The fulminant type manifests as respiratory distress and cyanosis shortly after the prodromal symptoms. In cases of acute pulmonary edema, symptoms include coughing, frothy pink sputum, tachycardia, and a drop or even disappearance of blood pressure. A few cases may result in sudden cardiac and respiratory arrest, leading to death after just a brief scream. The slow-onset type presents with milder respiratory and circulatory symptoms, sometimes even asymptomatic, and is only discovered when postpartum bleeding and incoagulable blood occur.
2. Systemic bleeding tendency: Some patients with amniotic fluid embolism survive the respiratory and circulatory failure phase but subsequently develop DIC. This manifests as a systemic bleeding tendency, primarily massive vaginal bleeding, along with mucosal, skin, stye bleeding, and hematuria, with the blood failing to clot. Notably, some cases of amniotic fluid embolism lack respiratory or circulatory symptoms and present primarily as uncontrollable postpartum vaginal bleeding. These cases should not be mistakenly attributed solely to uterine atony causing postpartum hemorrhage.
3. Multi-system organ injury: This condition affects all organs, with the kidneys being the most commonly damaged after the heart. Due to renal hypoxia, symptoms such as oliguria, anuria, hematuria, and azotemia may occur, potentially leading to death from renal failure. Brain hypoxia can cause dysphoria, spasms, and unconsciousness in affected individuals.The initial diagnosis should be made promptly based on typical clinical manifestations, and emergency treatment should be organized immediately. While performing rescue measures, necessary auxiliary examinations should be conducted, but treatment must not be delayed while awaiting test results, as this could lead to missing the critical window for intervention.
1. X-ray imaging: Typical findings include bilateral diffuse patchy infiltrates distributed around the hilar regions, accompanied by right heart enlargement and grade I atelectasis.
2. Detection of amniotic fluid components in pulmonary artery blood or inferior vena cava blood can confirm the diagnosis.
3. Laboratory findings supporting DIC diagnosis: ① Platelet count <100×109/L or progressive decline; ② Fibrinogen <1.5g/L; ③ Prothrombin time >15 seconds or exceeding the control group by more than 3 seconds; ④ Positive protamine paracoagulation (3P) test; ⑤ Clotting time by tube method >30 minutes (normal range: 8–12 minutes); ⑥ Blood smear showing fragmented red blood cells. A diagnosis of DIC requires at least three of the above positive findings. If fibrinogen cannot be measured, a simplified clotting time observation test can be used, with >16 minutes considered positive. The method is as follows: Collect 5ml of venous blood in a test tube and observe. If clotting occurs in 6–10 minutes, fibrinogen levels are normal; 11–15 minutes indicates fibrinogen >1.5g/L; 16–30 minutes indicates fibrinogen levels of 1.0–1.5g/L; and >30 minutes indicates fibrinogen <1.0g/L.
Sudden death cases can only be confirmed through autopsy. Examination of lung tissue sections may reveal amniotic fluid components in small pulmonary arteries and capillaries. If autopsy is not possible, immediate postmortem aspiration of right heart blood can be performed. The presence of amniotic fluid components or Sudan III-stained red fat globules can also confirm the diagnosis.
bubble_chart Treatment Measures
The key to successful resuscitation in amniotic fluid embolism lies in early diagnosis, prompt management, and the timely use of heparin and early intervention in the pregnant uterus. It can be summarized into the following aspects.
1. Anti-allergy: In cases of anaphylactic shock, large doses of corticosteroids should be administered, commonly hydrocortisone, with an immediate dose of 500mg, and generally 1000–2000mg daily via intravenous drip. However, corticosteroids can inhibit the function of the reticuloendothelial system, preventing the timely clearance of activated clotting factors and thereby exacerbating DIC. Therefore, caution should be exercised with repeated use, and it is preferable to administer this medication on the basis of heparin therapy.
2. Oxygen therapy: Continuous positive-pressure oxygen delivery should be prioritized, with at least mask oxygen therapy, as nasal catheter oxygen delivery is less effective. If available, a mechanical ventilator can be used to supply oxygen, which can alleviate pulmonary edema and improve hypoxia in the brain and other tissues.
3. Relief of pulmonary artery hypertension: Oxygen therapy only addresses alveolar oxygen pressure but not the low perfusion of pulmonary blood flow. Pulmonary artery hypertension must be relieved as early as possible to fundamentally improve hypoxia and prevent acute right heart failure, peripheral circulatory failure, and acute respiratory failure. Commonly used medications include:
(1) Aminophylline: Relieves pulmonary vascular spasm, dilates coronary arteries, and has diuretic effects, as well as relieving bronchial smooth muscle spasms. The dose is 0.25–0.5g added to 20ml of 10–25% glucose solution, administered intravenously.
(2) Papaverine: Dilates coronary, pulmonary, and cerebral blood vessels, making it an ideal drug for relieving pulmonary artery hypertension. The dose is 30–60mg added to 20ml of 25% glucose solution, administered intravenously.
(3) Atropine: Relieves pulmonary vascular spasm, inhibits bronchial secretion, and improves microcirculation. The dose is 0.5–1mg, administered intravenously every 10–15 minutes until symptoms improve.
(4) Phentolamine: Relieves pulmonary vascular spasm. The dose is 20mg added to 250ml of 10% glucose solution, administered via intravenous drip.
4. Anti-shock: The shock caused by amniotic fluid embolism is complex, involving factors such as allergy, pulmonary origin, cardiac origin, and DIC. Therefore, management must be comprehensive.
(1) Volume expansion: Shock is always accompanied by insufficient effective blood volume, so volume expansion should be initiated as early and quickly as possible. However, improper use can easily induce heart failure. If possible, a pulmonary artery catheter should be used to measure pulmonary capillary wedge pressure (PCWP), allowing for volume expansion while monitoring cardiac load. If PCWP measurement is unavailable, central venous pressure can guide fluid infusion. Regardless of the monitoring method, 5ml of blood should be drawn during catheter insertion for blood sedimentation tests, smear staining to identify amniotic fluid components, and relevant DIC laboratory tests. For volume expansion, dextran-40 (500–1000ml) is often used initially via intravenous drip. In cases of blood loss, fresh blood and balanced solutions should be supplemented.
(2) Correction of acidosis: Initially, 100–200ml of 5% sodium bicarbonate can be administered, or calculated using the formula: sodium bicarbonate (g) = (55 - measured CO2CP) × 0.026 × body weight (kg), with half to two-thirds of the calculated dose given first. Ideally, arterial blood gas and acid-base measurements should be performed to guide dosing based on imbalances.
(3) Adjustment of vascular tone: For patients with rapid and severe shock symptoms or unstable blood pressure despite adequate volume expansion, vasoactive drugs may be used. Dopamine (20–40mg in 500ml of glucose solution) is commonly administered via intravenous drip to ensure blood supply to vital organs.
5. Prevention and treatment of DIC: Once the diagnosis of amniotic fluid embolism is confirmed, anticoagulant therapy should be initiated, and heparin should be used as early as possible to inhibit intravascular coagulation and protect renal function. The initial dose of heparin is 1mg/kg (approximately 50mg), diluted in 100ml of normal saline and administered intravenously over 1 hour. The test tube coagulation time method can be used for monitoring to determine whether repeated administration is necessary. It is preferable to maintain the coagulation time at around 20 minutes. Amniotic fluid embolism can occur before, during, or after delivery. Be vigilant for severe postpartum metrorrhagia. The safest measure is to transfuse fresh blood on the basis of heparin administration and supplement with fibrinogen, platelet suspension, and fresh frozen plasma to replenish clotting factors and stop non-coagulating postpartum metrorrhagia.
6. Prevention of heart failure: Rapid-acting Rehmannia preparations can be used. Deslanoside (Cedilanid) 0.2–0.4 mg diluted in 20 ml of 25% glucose solution is administered intravenously, repeated every 4–6 hours if necessary, with a total daily dose <1.2 mg. Additionally, furosemide 40–80 mg is given intravenously to prevent and treat heart failure, which is crucial for improving the success rate of rescue efforts.
7. Prevention and treatment of multi-organ injury: In amniotic fluid embolism, apart from the lungs and heart, the kidneys are the next most commonly affected organs. To prevent renal failure, attention must be paid to renal perfusion during anti-shock treatment. Vasoconstrictors should not be used or should be used cautiously before adequate blood volume replacement. If urine output remains less than 17 ml per hour after blood volume is restored and blood pressure recovers, diuretics should be administered. If ineffective, this often indicates acute renal failure, and emergency measures such as hemodialysis should be initiated as soon as possible.
8. Timely and appropriate use of antibiotics to prevent infection.
9. Obstetric management: Prompt obstetric intervention is critical to the success of rescue efforts. If amniotic fluid embolism occurs before fetal delivery, efforts should focus on improving respiratory and circulatory function, preventing DIC, and managing shock. If the cervix is not fully dilated, a cesarean section should be performed to remove the underlying cause and prevent worsening of the condition. If the cervix is fully dilated and the fetal presenting part is below the ischial spines, forceps-assisted delivery may be performed. Close monitoring of uterine bleeding during and after the procedure is essential. If there is no bleeding, conservative treatment should continue. If uncontrollable postpartum hemorrhage with unclottable blood occurs, a hysterectomy should be performed decisively to control bleeding from placental detachment sites and prevent further entry of amniotic fluid debris into the circulation, which could exacerbate the condition. Opinions on the use of uterotonics vary. Opponents argue that enhancing uterine contractions may force residual amniotic fluid in the uterine wall into the maternal circulation, worsening the condition. However, it is well known that uterine contraction and retraction act as a biological vascular ligation, a key mechanism for hemostasis at placental detachment sites. Weighing the risks and benefits, uterotonics are still recommended to prevent postpartum hemorrhage. However, if oxytocin infusion is ongoing at the time of onset and delivery has not yet occurred, it should be stopped immediately.
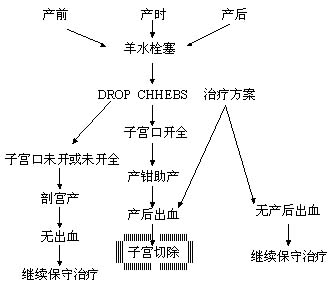
Figure 1: Obstetric management protocol for amniotic fluid embolism.
Paying attention to the following items will be beneficial for preventing amniotic fluid embolism.
1. During artificial rupture of membranes, avoid simultaneous membrane stripping to reduce damage to small blood vessels in the uterine cervix.
2. Avoid artificial rupture of membranes during uterine contractions or seasonal epidemics.
3. Strictly adhere to the indications for cesarean section, and protect open blood vessels on the uterine incision before puncturing the amniotic membrane during the procedure.
4. Follow the indications for oxytocin use.
5. Closely monitor conditions such as dead fetus and placental abruption.
6. Avoid birth injuries, uterine rupture, cervical lacerations, etc.




