| disease | Aortic Aneurysm |
| alias | Aortic Aneurysm |
Aortic aneurysm refers to the localized or diffuse abnormal dilation of the aortic wall, which compresses surrounding organs and causes symptoms. The main danger is the rupture of the aneurysm.
bubble_chart Etiology
The arterial wall's middle layer is rich in elastic fibers, which expand and contract with each heartbeat to transport blood. When the middle layer is damaged, the elastic fibers break and are replaced by fibrous scar tissue, causing the arterial wall to lose elasticity and become unable to withstand the impact of blood flow. As a result, the artery gradually dilates at the affected segment, forming an arterial aneurysm. Elevated arterial pressure contributes to the formation of arterial aneurysms. The main causes of aortic aneurysms are as follows:
(1) **Atherosclerosis**—This is the most common cause. Atherosclerotic plaques erode the aortic wall, damaging the middle layer and leading to degenerative changes in the elastic fibers. The arterial wall thickens due to atherosclerosis, compressing the nutrient vessels and causing nutritional impairment or rupture of these vessels, resulting in intramural hemorrhage. This condition is more common in elderly males, with a male-to-female ratio of approximately 10:1. It primarily affects the abdominal aorta, especially between the origin of the renal arteries and the iliac bifurcation.
(2) **Infection**—Syphilis is a notable cause, often eroding the thoracic aorta. Bacteremia from sepsis or endocarditis allows pathogens to reach the aorta via the bloodstream. Direct spread from adjacent abscesses or secondary infection on the basis of atherosclerotic ulcers can also lead to bacterial arterial aneurysms. The main pathogens include streptococci, staphylococci, and Salmonella, though such cases are relatively rare.
(3) **Cystic medial necrosis**—This is a relatively uncommon condition of unknown etiology. The elastic fibers in the aortic middle layer degenerate and are replaced by metachromatic acidic mucopolysaccharides. It mainly occurs in ascending aortic aneurysms and is more common in males. Hereditary diseases such as Marfan syndrome, Turner syndrome, and Ehlers-Danlos syndrome can also present with cystic medial necrosis, predisposing to dissecting aneurysms.(4) **Trauma**—Penetrating injuries directly damage the aorta, leading to aneurysms at any site. In cases of indirect trauma, forces often act on less mobile areas, such as the distal origin of the left subclavian artery or the root of the ascending aorta, rather than more mobile segments. Areas subjected to greater stress are more prone to aneurysm formation.
(5) **Congenital causes**—These primarily include aortic sinus aneurysms.
(6) **Others**—Conditions such as giant cell arteritis, Behçet’s disease, and Takayasu arteritis may also contribute.
bubble_chart Pathological Changes
According to the structure, aortic aneurysms can be divided into: ① True aortic aneurysm: The sac of the aneurysm is composed of one or more layers of the aortic wall; ② False aortic aneurysm: Due to trauma, infection, or other reasons, blood leaks from the aorta into the surrounding tissues, and the blood clot, its organized material, fibrous tissue, and the aortic wall together form the wall of the aneurysm. ③ Dissecting aortic aneurysm. After the intima or media of the aorta tears, the impact of blood flow causes the media to gradually separate into layers, accumulating blood and bulging in the separated cavity, which may also form a double-lumen structure with the aortic cavity (Figure 3). Based on morphology, aortic aneurysms can be classified into: ① Saccular aortic aneurysm: The aneurysm involves part of the aortic circumference, appearing sac-like, possibly with a neck, and asymmetrically protruding. ② Fusiform aortic aneurysm: The aneurysm involves the entire aortic circumference. Traumatic aneurysms are often saccular, while atherosclerotic aneurysms are typically fusiform. According to the location of occurrence, aortic aneurysms can be divided into: ① Ascending aortic aneurysm, often involving the aortic root; ② Aortic arch aneurysm; ③ Descending aortic aneurysm or thoracic aortic aneurysm, originating distal to the left subclavian artery; ④ Abdominal aortic aneurysm, usually distal to the renal arteries. Proximal ascending aortic aneurysms involving the aortic sinus are often congenital, followed by Marfan syndrome, syphilis, and infection; ascending aortic aneurysms are mainly caused by atherosclerosis, cystic medial necrosis, and syphilis; descending aortic aneurysms and abdominal aortic aneurysms are primarily due to atherosclerosis. Most aortic aneurysms are single, with very few being two or multiple. As the disease progresses, aortic aneurysms may experience: ① Rupture: The weakened wall of the aneurysm gradually expands under continuous blood flow impact, eventually perforating and causing hemorrhage. ② Mural thrombus formation: Blood flow slows in the bulging area of the aneurysm, forming vortices. If the inner surface of the aneurysm wall is rough, thrombus formation is likely, and thrombus detachment can lead to embolism. ③ Secondary infection: Secondary infection further weakens the aneurysm wall, making it more prone to rupture. Sometimes, the aneurysm repeatedly bleeds small amounts into the surrounding tissues, accumulating a large amount of fibrous tissue around the aneurysm, forming a capsule, which may provide protective effects to prevent rupture.
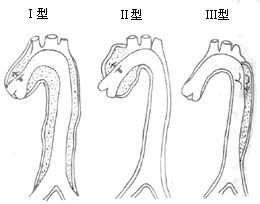
Figure 3 Schematic diagram of aortic dissection aneurysm classification.
bubble_chart Clinical Manifestations
The symptoms of an aortic aneurysm are caused by the aneurysm compressing, pulling, or eroding surrounding tissues, depending on the size and location of the aortic aneurysm. When a thoracic aortic aneurysm compresses the superior vena cava, it can cause venous distension in the face, neck, and shoulders, and may lead to edema; compression of the trachea and bronchi can cause cough and shortness of breath; compression of the esophagus can result in difficulty swallowing; and compression of the recurrent laryngeal nerve can lead to hoarseness. A thoracic aortic aneurysm located in the ascending aorta may deform the aortic valve annulus, causing separation of the valve leaflets and resulting in aortic valve insufficiency, accompanied by corresponding murmurs. Most cases progress slowly with few symptoms, but if the onset is sudden, it can lead to acute pulmonary edema. Thoracic aortic aneurysms often cause pain, and sudden intensification of pain may indicate a risk of rupture. An aortic arch aneurysm compressing the left innominate vein can cause higher venous pressure in the left arm compared to the right. An ascending aortic aneurysm may erode the sternum and costal cartilage, protruding as a pulsatile mass on the anterior chest; a descending aortic aneurysm may erode the transverse processes of the thoracic vertebrae and ribs, even protruding on the back; erosion of bone at any site can cause pain. Rupture of a thoracic aortic aneurysm into the bronchi, trachea, pleural cavity, or pericardium can be fatal.
Abdominal aortic aneurysms are common and may be asymptomatic. Since the primary cause is atherosclerosis, symptoms of renal, cerebral, or coronary atherosclerosis are often present. The first noticeable sign is usually a pulsatile mass in the abdomen. The most common symptom is abdominal pain, typically located around the umbilicus or in the mid-upper abdomen, and may also involve the back. The onset and progression of pain indicate enlargement of the aneurysm or minor bleeding. Severe, persistent pain radiating to the back, pelvis, perineum, or lower limbs, or significant tenderness over the mass, are signs of rupture. Abdominal aortic aneurysms often rupture into the left retroperitoneal space, less commonly into the abdominal cavity, and occasionally into the duodenum or vena cava. Shock often follows rupture. Unless the patient is excessively obese, the pulsatile mass is usually easy to palpate, typically between the umbilicus and the pubis. Sometimes a systolic murmur can be heard over the mass, and a few cases may also present with a tremor. Palpation of an aortic aneurysm, especially when tender, must be done with caution to avoid precipitating rupture. Compression of the iliac vein by an abdominal aortic aneurysm can cause lower limb edema; compression of the spermatic vein may lead to local varicose veins; and compression of a ureter can result in hydronephrosis, pyelonephritis, and impaired renal function.
bubble_chart DiagnosisThe discovery of a thoracic aortic aneurysm relies not only on symptoms and signs but also on X-ray examination. On posteroanterior and lateral radiographs, an enlarged aortic shadow can be observed, from which the size, location, and morphology of the lesion can be estimated. Under fluoroscopy, the expansile pulsation of the aortic aneurysm may be visible, though pulsation may be less apparent if thrombosis has occurred within the aneurysm. Aortic aneurysms must be differentiated from solid masses attached to the aorta, as the latter exhibit transmitted pulsations. Aortography can aid in this differentiation (Figures 1 and 2). Echocardiography can detect aneurysms in the ascending aorta, where the aortic diameter is enlarged. Computed tomography (CT) is also useful for diagnosis.
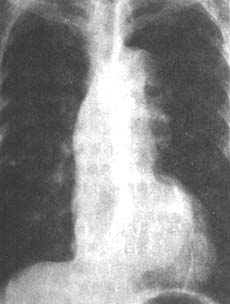
**Figure 1** Posteroanterior X-ray of a thoracic aortic aneurysm
shows bulging of the ascending aortic border, widening of the aorta, broadening of the esophageal aortic impression, and significant irregularity of the descending aortic contour with multiple aneurysmal formations.
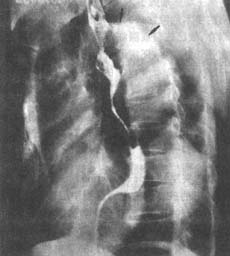
**Figure 2** Left anterior oblique X-ray of a thoracic aortic aneurysm
reveals a wavy contour along the upper edge of the aortic arch (↑). The esophagus follows the tortuous aorta, and the undulating margins represent adjacent irregular aortic dilation and aneurysms.
Abdominal aortic aneurysms are often discovered upon palpation of a pulsatile abdominal mass. However, palpable aortic pulsations in the abdomen do not necessarily indicate an aneurysm, as a normal abdominal aorta may be easily felt in thin individuals or those with lumbar lordosis. A systolic vascular murmur heard in the abdomen may arise from grade I stenosis of the renal, splenic, or mesenteric arteries and does not always originate from an aortic aneurysm, warranting caution. Ultrasound examination is crucial for definitive diagnosis, and many cases are now detected during routine ultrasound screenings, significantly increasing the diagnostic detection rate compared to the past. The examination reveals an increased aortic diameter and widened fluid-filled segment between the anterior and posterior walls. If thrombosis is present, the widened segment may be less distinct, but the synchronous pulsation of the aneurysm walls with the heartbeat persists, and the external aortic diameter remains enlarged. Ultrasound can accurately determine the size of the lesion (with precision up to 2–3 mm), its extent, morphology, and the presence of intraluminal thrombus. CT scanning is equally useful, particularly for detecting intraluminal thrombus and wall calcifications. It also delineates the relationship between the aneurysm and adjacent structures such as the renal arteries, retroperitoneal space, and spine. MRI is as valuable as CT and abdominal ultrasound for assessing aneurysm size and its relation to the renal and iliac arteries. However, MRI's primary drawbacks are the time-consuming image analysis and high cost. Aortography also aids in localization, though intraluminal thrombus may affect the assessment of lesion severity. Nevertheless, aortography is still recommended for cases with unclear diagnoses, hypertensive patients with concurrent renal artery involvement, suspected obstructions or aneurysmal lesions, and those undergoing surgical planning.
bubble_chart Treatment Measures
Surgical treatment, including resection of the aneurysm and artificial or homologous vascular grafting, can be performed for those with unresectable aneurysms by wrapping the aneurysm. Currently, the surgical mortality rate for abdominal aortic aneurysms is less than 5%, but for elderly patients with heart, brain, kidney, or other visceral damage, the surgical mortality can exceed 25%. The surgical mortality rate for thoracic aortic aneurysms is 30%, with the highest surgical risk associated with aortic arch aneurysms. Few patients survive a ruptured aneurysm without surgery, so immediate surgery is required for those with ruptured or impending rupture. Patients with bacterial aneurysms also require long-term antibiotic therapy. Elective surgery is recommended for aortic aneurysms measuring 6 cm or larger or exceeding 4 cm. For aortic aneurysms between 4 and 6 cm, close monitoring is advised, and immediate surgery is necessary if there are signs of enlargement or impending rupture.
The main aneurysm causes an increase in intravascular pressure, leading to progressive enlargement. If left untreated, it will eventually rupture, with the likelihood of rupture increasing as the aneurysm grows larger. Embolism is another complication. Statistics show that without surgical treatment, 90% of thoracic aortic aneurysms result in death within five years, and three-quarters of abdominal aortic aneurysms lead to death within the same timeframe.




