| disease | Mitral Stenosis |
| alias | Mitral Stenosis |
The vast majority of mitral stenosis cases are sequelae of wind-dampness heat. A very small number are congenital stenosis or senile calcification of the mitral valve annulus or subannular region. Two-thirds of mitral stenosis patients are female. Approximately 40% of rheumatic heart disease patients present with pure mitral stenosis, exhibiting a mitral facies.
bubble_chart Pathogenesis
The normal mitral valve is soft in texture, with a valve orifice area of approximately 4–6 cm2. When the valve orifice area decreases to 1.5–2.0 cm2, it is classified as grade I stenosis; 1.0–1.5 cm2 as grade II stenosis; and <1.0 cm2 as grade III stenosis. The primary pathophysiological change after mitral stenosis is the restriction of diastolic blood flow from the left atrium to the left ventricle, leading to abnormally elevated left atrial pressure and an increased pressure gradient between the left atrium and left ventricle to maintain normal cardiac output. The rise in left atrial pressure can increase pulmonary venous and pulmonary capillary pressures, subsequently causing dilation and congestion. At this stage, patients may show no obvious symptoms at rest, but during physical activity, due to accelerated blood flow, pulmonary venous and pulmonary capillary pressures rise further, immediately leading to dyspnea, cough, cyanosis, or even acute pulmonary edema. Chronic overload of pulmonary circulation blood volume can result in elevated pulmonary artery pressure. Long-term pulmonary hypertension causes pulmonary arterioles to spasm and harden, leading to right ventricular hypertrophy and dilation, which may eventually progress to right ventricular failure. At this point, pulmonary artery pressure decreases somewhat, pulmonary circulation blood flow reduces, and pulmonary congestion is alleviated.
In pure mitral stenosis, left ventricular end-diastolic pressure and volume are normal. Most mitral stenosis patients exhibit an increased left ventricular ejection fraction during exercise and a reduced end-systolic volume. About one-quarter of severe mitral stenosis patients develop left ventricular dysfunction, manifested by a decreased ejection fraction and other indices of systolic function, likely due to chronic reduction in preload. Most mitral stenosis patients have a resting cardiac output within the normal range, but the increase in cardiac output during exercise is below normal. A few with severe stenosis have a resting cardiac output below normal, and their cardiac output does not increase or even decreases during exercise, primarily due not only to mitral stenosis but also to impaired function of both ventricles. Additionally, due to left atrial enlargement, maintaining normal electrical activity becomes difficult, often resulting in atrial fibrillation. Rapid atrial fibrillation with a high ventricular rate can elevate pulmonary capillary pressure, exacerbating pulmonary congestion or triggering pulmonary edema.bubble_chart Pathological Changes
The pathological changes initially involve inflammation, edema, and vegetation formation at the junction and base of the valve membrane. Due to fibrosis and/or calcium deposition, the valve leaflets become extensively thickened, adhered, and fused with shortened chordae tendineae, leading to stiffening of the leaflets. This results in deformation and stenosis of the valve orifice, which, when severe, becomes a slit-like opening. Based on the severity of the lesions, it can be classified into the membranous type and the funnel-shaped type. In the membranous type, the main valve body is either unaffected or minimally affected, retaining some mobility. In the funnel-shaped type, the valve leaflets are significantly thickened and fibrotic, with marked adhesion and shortening of the chordae tendineae and papillary muscles, causing the entire valve membrane to harden into a funnel shape and severely restricting movement. This condition is often accompanied by varying degrees of regurgitation. Calcification of the leaflets further exacerbates stenosis and may lead to thrombosis and embolism. In congenital mitral stenosis, the valve leaflets are thickened, the commissures are fused, the chordae tendineae are thickened or shortened, and the papillary muscles are hypertrophied or fibrotic. There may be a supravalvular stenotic ring or subvalvular fibrous bands. The most characteristic feature is the parachute deformity of the mitral valve, where a single papillary muscle connects to the chordae tendineae of both leaflets, causing the entire valve membrane to open like a parachute.
(I) Symptoms Normally, it can take up to 10 years from the initial wind-dampness-induced carditis to the appearance of obvious symptoms of mitral stenosis; thereafter, there is a gradual loss of activity over 10 to 20 years.
1. Dyspnea Exertional dyspnea is the earliest symptom, primarily due to reduced lung compliance. As the disease progresses, dyspnea can occur during daily activities, along with orthopnea. Triggers such as exertion, emotional stress, respiratory infections, sexual intercourse, pregnancy, or rapid atrial fibrillation can induce acute pulmonary edema.
2. Cough Mostly occurs during nighttime sleep and after exertion. It is often a dry cough; with concurrent bronchitis or lung infection, mucus-like or purulent sputum may be coughed up. Significant left atrial enlargement compressing the bronchi can also cause cough.
3. Hemoptysis ① Blood-streaked sputum or bloody sputum, related to bronchitis, lung infection, pulmonary congestion, or capillary rupture; often accompanied by nocturnal paroxysmal dyspnea; in advanced stages of mitral stenosis, hemoptysis may also occur due to pulmonary infarction. ② Massive hemoptysis results from a sudden increase in left atrial pressure leading to rupture of bronchial veins. This is more common in the early stages of mitral stenosis, in patients with only grade I or grade II pulmonary stirred pulse elevation. ③ Pink frothy sputum, caused by capillary rupture, is characteristic of acute pulmonary edema.
4. Chest Pain About 15% of mitral stenosis patients experience chest pain, likely due to increased tension in the hypertrophied right ventricular wall combined with reduced cardiac output causing right ventricular ischemia. This can be relieved after mitral valve separation or dilation surgery.
6. Other Symptoms Left atrial enlargement and left pulmonary stirred pulse dilation can compress the left recurrent laryngeal nerve, causing hoarseness; significant left atrial enlargement can compress the esophagus, leading to dysphagia; right ventricular failure can cause symptoms such as loss of appetite, abdominal distension and fullness, and nausea.
(II) Characteristics
1. Cardiac Auscultation A low-pitched rumbling murmur in the mid-to-late diastolic phase at the apex, crescendo in nature, localized, more pronounced in the left lateral decubitus position, and may be accompanied by diastolic tremor. The first heart sound at the apex is accentuated and snapping. In 80–85% of patients, a mitral opening snap (OS) can be heard at the left sternal border 3–4 intercostal space or the medial apex. This sound follows closely after the second heart sound, is high-pitched, short, and loud, and is more pronounced during expiration. It is caused by the tremor of the main valve (anterior mitral leaflet) of the diaphragm-type valve opening. The presence of a snapping first heart sound and mitral opening snap strongly suggests mitral stenosis and indicates that the valve still has some flexibility and mobility, aiding in the diagnosis of diaphragm-type mitral stenosis and having some significance in determining surgical treatment methods. Due to pulmonary stirred pulse hypertension, there may be an accentuated and split second heart sound of the pulmonary stirred pulse valve. In severe pulmonary stirred pulse hypertension, a high-pitched, decrescendo early-to-mid diastolic murmur can be heard at the left sternal border 2–4 intercostal space, blowing in nature, radiating along the left sternal border to the tricuspid area, and intensifying during inspiration. This is due to the dilation of the pulmonary stirred pulse and its annulus, causing relative pulmonary stirred pulse valve insufficiency (Graham-Steell murmur). Sometimes, an early systolic click of the pulmonary stirred pulse valve can also be heard, more pronounced during expiration and diminished during inspiration. In severe mitral stenosis patients, due to pulmonary stirred pulse hypertension and right ventricular enlargement, the tricuspid annulus dilates, leading to relative tricuspid insufficiency. During right ventricular contraction, some blood flows back into the right atrium through the tricuspid valve, resulting in a blowing pansystolic murmur at the tricuspid area, radiating to the apex and more pronounced during inspiration.
2. Other signs: Mitral facies is seen in patients with severe mitral stenosis. Due to reduced cardiac output, the patient exhibits a purplish-red discoloration on both cheeks and grade I cyanosis on the lips. Cyanosis is also observed in the extremities. In cases of mitral stenosis occurring during childhood, a bulging precordium may be noted, with the left nipple displaced upward and outward, along with a systolic heaving impulse at the left sternal border. Patients with grade II or higher stenosis demonstrate an enlarged cardiac dullness extending leftward at the third intercostal space along the left sternal border, indicating pulmonary stirred pulse and right ventricular enlargement. Prominent jugular venous pulsation suggests the presence of severe pulmonary stirred pulse hypertension.
bubble_chart Auxiliary Examination
(1) X-ray Examination The earliest changes are a prominent left atrial curvature along the left heart border, a protruding pulmonary artery trunk, and widened pulmonary veins. Barium fluoroscopy in the right anterior oblique view may reveal an enlarged left atrium compressing the esophagus. In severe cases, the left atrium and right ventricle are significantly enlarged. The posteroanterior view shows a double shadow along the right heart border, deepened hilar shadows, and a smaller aortic arch. The left ventricle is generally not enlarged. When left atrial pressure reaches 2.7 kPa (20 mmHg), Kerley B lines may be visible in the middle and lower lung fields. After prolonged pulmonary congestion, hemosiderin deposits may appear as scattered dot-like shadows in the lower lung fields. Mitral valve calcification is common in elderly patients and is also not uncommon in younger adults (Figures 1, 2).
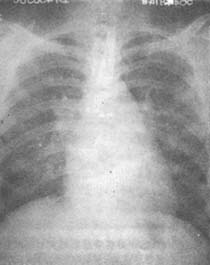
Figure 1: Rheumatic Heart Disease—Mitral Stenosis
Posteroanterior chest X-ray: The mid-segment of the left heart border is full, and the left atrium protrudes above the right atrium on the right border, forming a double arch.
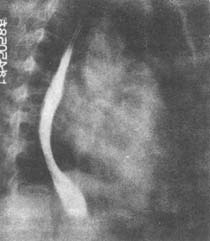
Figure 2: Rheumatic Heart Disease—Mitral Stenosis
Right anterior oblique chest X-ray: The lower esophagus is displaced posteriorly due to compression by the enlarged left atrium, and the pulmonary artery cone is prominent.
(2) Electrocardiogram (ECG) Examination In grade I mitral stenosis, the ECG may be normal. The characteristic finding is a widened and notched P wave, indicating left atrial enlargement. With pulmonary hypertension, right ventricular hypertrophy and right axis deviation may be seen. In advanced stages, atrial fibrillation is common (Figure 3).
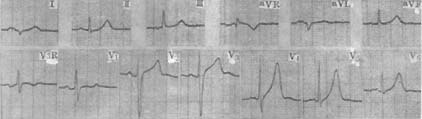
Figure 3: Rheumatic Heart Disease—Mitral Stenosis
(3) Echocardiography This is the most sensitive and specific non-invasive diagnostic method. It is highly valuable for determining valve orifice area and transvalvular pressure gradients, assessing disease severity, deciding on surgical approaches, and evaluating treatment outcomes. Two-dimensional echocardiography shows thickened mitral valve leaflets with reduced mobility. During diastole, the anterior leaflet bulges forward in a balloon-like shape, and the distance between the tips of the anterior and posterior leaflets is significantly reduced, with a decreased orifice area. M-mode echocardiography reveals slowed diastolic filling rates, loss of the normal biphasic pattern, and a slow descent after the E peak. The anterior and posterior mitral leaflets move in the same direction during diastole (so-called "doming" or "hockey stick" appearance). Left atrial enlargement, right ventricular hypertrophy, and widening of the right ventricular outflow tract are also observed. Doppler ultrasound demonstrates slow and decelerated blood flow across the mitral valve (Figures 4, 5, 6).
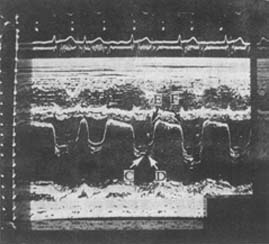
Figure 4: M-Mode Echocardiogram of Mitral Stenosis
The mitral valve curve shows a "doming" pattern with irregular wave morphology due to atrial fibrillation.
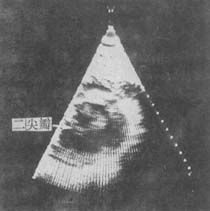
Figure 5A: Parasternal Long-Axis View of Mitral Stenosis
Diastolic mitral valve orifice is restricted, and the leaflets are thickened.
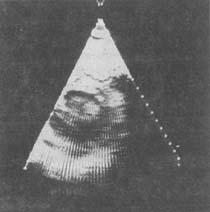
Figure 5B: Parasternal Short-Axis View of Mitral Stenosis
(Shows systolic phase)

Figure 6A: Apical Long-Axis View of Mitral Stenosis
Diastolic doming of the anterior mitral leaflet, with left atrial and left ventricular enlargement.
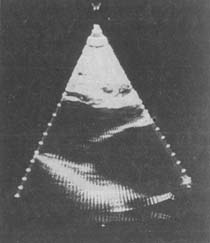
Figure 6B Fan-shaped echocardiogram long-axis view of mitral stenosis
showing systole
(4) Radionuclide examination: Radionuclide blood pool imaging shows left atrial enlargement, increased tracer concentration, prolonged transit time, and normal left ventricular size.
In pulmonary hypertension, dilation of the pulmonary artery trunk and right ventricle can be observed.
(5) Right heart catheterization: Increased pressures in the right ventricle, pulmonary artery, and pulmonary capillaries, elevated pulmonary vascular resistance, and reduced cardiac output. After transseptal puncture, left atrial pressure can be directly measured. In early mitral stenosis, the diastolic transvalvular pressure gradient is normal, but as the condition worsens, the pressure gradient increases, and the left atrial pressure curve during contraction shows a tall a wave.
1. Medical History and Symptoms:
The earliest symptom is paroxysmal nocturnal dyspnea, which may progress to orthopnea in severe cases. In extreme cases, pulmonary edema may occur, accompanied by cough and pink frothy sputum, often worsening during sleep or after activity. Symptoms may also include sputum production, blood-streaked sputum, and hemoptysis. As the condition progresses, lower limb edema and oliguria may develop, at which point dyspnea may alleviate.
2. Physical Examination Findings:
Mitral facies and grade I cyanosis of the lips are observed. The precordium is prominent, and a diastolic thrill may be palpated at the apex. The cardiac border is enlarged to the left at the third intercostal space. S1 at the apex is accentuated and snapping in quality. An opening snap may be heard between the left sternal border (III–IV intercostal spaces) and the upper medial apex. If the valve leaflets lose elasticity, the accentuated S1 and opening snap may disappear. A mid-to-late diastolic rumbling murmur, crescendo in nature, is audible at the apex, becoming more pronounced in the left lateral decubitus position, at the end of respiration, and after activity. The pulmonary component of the second heart sound (P2) is accentuated and split. A short, early diastolic blowing murmur (Graham-Steell murmur) is heard at the pulmonary area (left sternal border, II–III intercostal spaces), intensifying with deep inspiration.
3. Auxiliary Examinations:
X-ray reveals prominence of the pulmonary artery, enlargement of the left atrium and right ventricle, elevation of the left main bronchus, and an esophageal indentation due to left atrial pressure. Increased and thickened vascular markings are seen in the upper lung fields, with Kerley's B lines visible at the costophrenic angles. ECG shows a widened P wave (>0.11s) with notching and right ventricular hypertrophy. In the late stage (third stage), atrial fibrillation may appear. Echocardiography demonstrates thickening, adhesion, and calcification of the mitral valve, along with stenosis, enlargement of the left atrium and right ventricle, and possible atrial thrombi. Doppler ultrasound reveals a diastolic turbulent flow spectrum below the mitral valve.
4. Differential Diagnosis:
It should be differentiated from mitral stenosis caused by other diseases.
bubble_chart Treatment Measures
(1) Treatment in the compensatory phase: Appropriately avoid excessive physical labor and strenuous exercise to protect cardiac function; for patients with Rheumatic heart disease, active prevention of streptococcal infection, wind-dampness activity, and infective endocarditis should be emphasized.
(2) Treatment in the decompensatory phase: For patients with clinical symptoms, oral diuretics and sodium restriction are recommended. When right heart failure is evident or rapid atrial fibrillation occurs, digitalis preparations can alleviate symptoms and control ventricular rate. For patients with persistent atrial fibrillation for less than one year, pharmacological or electrical cardioversion should be considered. Long-term heart failure accompanied by atrial fibrillation may require anticoagulant therapy to prevent thrombosis and systemic embolism.
The key to treatment is relieving mitral stenosis and reducing the transvalvular pressure gradient. Commonly used surgical methods include:
1. Percutaneous balloon mitral valvuloplasty. This is an interventional cardiac catheterization technique indicated for pure mitral stenosis. This method can enlarge the mitral valve area to 2.0cm2 or more, significantly reduce the transvalvular pressure gradient and left atrial pressure, increase the cardiac index, and effectively improve clinical symptoms. Percutaneous balloon mitral valvuloplasty does not damage the subvalvular structures, and skilled operators can also avoid complications. Moreover, it does not require thoracotomy, is relatively safe, causes minimal patient injury, and allows for rapid recovery, with confirmed short-term efficacy.
2. Mitral commissurotomy: There are closed and open methods. The closed method often involves entering through the left ventricle using a dilator, which is most effective for the membranous type. The surgical indications are patients aged no more than 55 years, with cardiac function class 2–3, no wind-dampness activity or infective endocarditis in the past six months, no atrial thrombus on preoperative examination, and no or only grade I mitral regurgitation or aortic valve disease with no left ventricular enlargement. For pregnant patients requiring surgery, it is best performed within the first six months of pregnancy. For grade II or grade III mitral regurgitation, suspected atrial thrombus formation, grade III valvular calcification, or significant fusion and shortening of chordae tendineae, open commissurotomy should be performed.
3. Prosthetic valve replacement: The indications are cardiac function class 3–4, with significant mitral regurgitation and/or aortic valve disease and left ventricular enlargement; severe valvular calcification making separation and repair impossible; or stenosis caused by calcified atheroma. Mechanical or biological valves are commonly used. Mechanical valves are durable and not prone to calcification or infection but require lifelong anticoagulation therapy; they are contraindicated in patients with peptic ulcer or bleeding disorders. Biological valves do not require anticoagulation but may fail due to infective endocarditis or valvular calcification or mechanical injury after several years.
(1) Arrhythmia Atrial arrhythmias are the most common, initially presenting as atrial premature beats, followed by atrial tachycardia, atrial flutter, paroxysmal atrial fibrillation, and eventually persistent atrial fibrillation. The pathological basis for the persistence of atrial fibrillation lies in the left atrial enlargement caused by elevated left atrial pressure and the fibrosis of the left atrial wall due to wind-dampness inflammation. Atrial fibrillation reduces cardiac output and may induce or exacerbate heart failure. After the onset of atrial fibrillation, the presystolic accentuation of the diastolic rumbling murmur in the apical region may disappear. During rapid atrial fibrillation, the diastolic rumbling murmur in the apical region may diminish or disappear, only to become apparent again or reappear when the heart rate slows.
(2) Congestive Heart Failure and Acute Pulmonary Edema Congestive heart failure occurs in 50–75% of patients and is the leading cause of death in mitral stenosis. Respiratory infections are common triggers for heart failure, while in female patients, pregnancy and childbirth also frequently induce heart failure. Acute pulmonary edema is a severe and emergent complication of grade III mitral stenosis, often precipitated by intense physical activity, emotional stress, infection, sudden tachycardia, or rapid atrial fibrillation, and is more likely to occur during pregnancy and childbirth. Under these conditions, the ventricular rate increases significantly, shortening the left ventricular diastolic filling time, while pulmonary circulation volume rises. The left atrial pressure markedly increases, elevating pulmonary capillary pressure and causing plasma to leak into interstitial spaces or alveoli, leading to acute pulmonary edema.
(3) Embolism Cerebral embolism is the most common, but emboli may also occur in the limbs, intestines, kidneys, spleen, and other organs. Most emboli originate from the enlarged left atrial appendage in patients with atrial fibrillation. Emboli from the right atrium may cause pulmonary embolism or pulmonary infarction.
(4) Pulmonary Infection Patients with this condition often have elevated pulmonary venous pressure and pulmonary congestion, making them prone to pulmonary infections. Once pulmonary infection occurs, it often exacerbates or triggers heart failure.
(5) Subacute Infective Endocarditis This is relatively rare.
The detection of a rumbling diastolic murmur at the cardiac apex accompanied by left atrial enlargement is sufficient to diagnose mitral stenosis, and echocardiography can confirm the diagnosis. Clinically, mitral stenosis should be differentiated from the following conditions with apical diastolic murmurs:
(1) Acute rheumatic carditis: A high-pitched, soft early diastolic murmur is heard at the cardiac apex, which varies significantly from day to day. The murmur may disappear after the rheumatic activity is controlled. This is due to ventricular dilation causing relative mitral stenosis, known as the Carey-Coombs murmur.
(2) "Functional" mitral stenosis: This occurs in conditions where the left ventricle is enlarged due to various causes, mitral valve flow increases, or the mitral valve is impacted by regurgitant blood from the aorta during diastole, such as in large left-to-right shunts (e.g., patent ductus arteriosus or ventricular septal defect) or aortic regurgitation. This murmur is shorter in duration, lacks an opening snap, is relatively soft, diminishes with amyl nitrite inhalation, and intensifies with vasopressor administration.
(3) Left atrial myxoma: The most common primary cardiac tumor. Clinical symptoms and signs resemble mitral stenosis but are intermittent and change with body position. Typically, no opening snap is heard, but a tumor plop may be audible. Atrial fibrillation is rare, but recurrent peripheral arterial embolism is common. Echocardiography reveals a cloud-like echo behind the mitral valve during both systole and diastole. Cardiac catheterization shows significantly elevated left atrial pressure, and selective angiography demonstrates a filling defect in the left atrium. The latter is now rarely used due to the risk of tumor embolism.
(4) Tricuspid stenosis: A low-pitched rumbling diastolic murmur is heard at the lower left sternal border, which intensifies during inspiration (due to increased venous return) and weakens during expiration. In sinus rhythm, the jugular venous a-wave is prominent. In contrast, the diastolic murmur of mitral stenosis is localized to the apex and does not change or weakens with inspiration. Echocardiography can confirm the diagnosis.
(5) Primary pulmonary hypertension: More common in female patients, with no apical diastolic murmur or opening snap. The left atrium is not enlarged, and pulmonary capillary wedge pressure and left atrial pressure are normal.





