| disease | Disseminated Intravascular Coagulation |
| alias | DIC, Dessiminated Intiavascular Coagulation, Consumptive Coagulopathy, Defibrination Syndrome, Intravascular Coagulation-defibrinolysis Syndrome |
Disseminated intravascular coagulation (DIC) is an acquired disorder that occurs during the pathological progression of many diseases. Due to coagulation in small blood vessels, extensive microthrombi form, consuming large amounts of clotting factors and subsequently activating fibrinolysis, leading to severe and widespread systemic bleeding. This condition is also referred to by various names, such as: ①defibrination syndrome; ②consumptive coagulopathy; ③intravascular coagulation with fibrinolysis syndrome. Clinically, DIC may present with a series of major manifestations, including bleeding, shock, organ damage, and hemolysis. The disease tends to be highly dangerous with a high mortality rate.
bubble_chart Etiology
There are many disease causes that can lead to DIC. According to an analysis of domestic data, infections are the most common, accounting for more than one-third of the total cases, followed by malignant tumors (including acute promyelocytic leukemia), with these two categories combined accounting for about two-thirds of the disease causes. Extensive surgical procedures, tissue injury, obstetric accidents, and extracorporeal circulation are also common disease causes of DIC.
The pathophysiological mechanisms by which various disease causes trigger DIC are not entirely the same. The main mechanisms leading to DIC are as follows:
(1) **Infections** Both Gram-negative and Gram-positive bacterial sepsis can cause DIC, but Gram-negative bacteria are more common, such as *Escherichia coli*, *Proteus*, *Pseudomonas aeruginosa*, and *Salmonella typhi*. Gram-positive bacteria include *Staphylococcus aureus*, *Bacillus anthracis*, *Staphylococcus aureus*, *Streptococcus hemolyticus*, and *Clostridium*. Non-bacterial infections are less common, such as viruses, rickettsiae, protozoa, spirochetes, and fungal infections. The pathogenesis of bacterial infections mainly involves factors from the infection itself and the endotoxins produced. After bacterial infection, vascular endothelial cell injury can release large amounts of tissue factor into the bloodstream, promoting coagulation. The activation of the complement system also plays a role in coagulation, fibrinolysis, and the kinin system. Regarding endotoxins, experiments have shown that adding Gram-negative bacterial endotoxins to blood in vitro can induce tissue factor activity on the monocyte membrane. If rabbits are treated with large doses of alkylating agents to deplete monocytes before endotoxin injection, DIC does not occur. Endotoxin contact with vascular endothelial cells can also generate tissue factor activity. However, it has also been found that the ratio of peptidoglycan (a DIC-inducing glycopeptide) to teichoic acid in the cell wall of Gram-positive bacteria is related to the occurrence of DIC. This demonstrates that the pathological mechanisms of DIC in infections are complex and multifaceted. Bradykinin has a strong vasodilatory effect, contributing to hypotension and shock during infections.
(2) **Malignant Tumors** Among cancers, pancreatic, renal, prostate, and bronchial cancers are more commonly associated with DIC. Acute promyelocytic leukemia is also prone to complicating DIC. In cancer cases, DIC is particularly likely to occur in patients with extensive metastasis or large areas of tissue necrosis. This is because tumor cells secrete large amounts of mucin, tissue factor, procoagulant substances, and proteolytic enzymes, which promote coagulation and trigger DIC. Trousseau syndrome is a manifestation of chronic DIC in malignant tumors, characterized by recurrent migratory arterial and venous thrombosis, and may even be the initial symptom.(3) **Obstetric Accidents** These include amniotic fluid embolism, placental abruption, hypertonic saline-induced late abortion, pregnancy toxemia, dead fetus retention, uterine rupture, and cesarean section, all of which can lead to DIC. The main mechanism involves the entry of large amounts of tissue factor from amniotic fluid and placental tissue into the circulation, promoting blood coagulation. Additionally, hypercoagulability and abnormal changes in blood vessels and blood flow may also contribute to the pathogenesis.
(4) Others ① Severe head injury complicated by DIC may occur due to coagulation-active factors entering the bloodstream through the disrupted blood-brain barrier, promoting blood coagulation. ② Poisonous snake bite causing DIC involves not only the release of large amounts of tissue factors after tissue injury, which enter the blood and promote coagulation, but also the secretion of substances from the snake venom itself that can convert fibrinogen into fibrin. ③ Immune diseases such as systemic lupus erythematosus and graft rejection reactions leading to DIC are primarily caused by abnormal immune mechanisms in the disease, resulting in widespread vascular endothelial cell injury, and complement activation is related to the promotion of coagulation mechanisms. ④ Liver diseases such as acute liver necrosis, cirrhosis, and other cases with severe liver function impairment are also prone to DIC. The reasons include not only vascular endothelial injury and the influence of procoagulant substances similar to the above but also the weakened function of phagocytosis and clearance of procoagulant substances in liver diseases. ⑤ Elevated body temperature, acidosis, shock, and hypoxia causing vascular endothelial cell injury can induce or exacerbate DIC. In hemolytic diseases or hemolytic reactions, red blood cells can also trigger procoagulant substances to induce or worsen DIC.
In recent years, the role of platelet-activating factor (PAF) in the pathogenesis of DIC has gained attention (Figure 1).
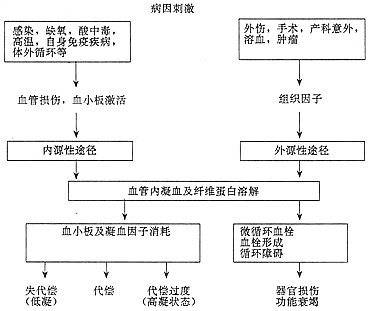
Figure 1: Disease causes and pathological changes in disseminated intravascular coagulation.
bubble_chart Pathological Changes
In 90% of autopsy cases of DIC, microthrombi and fibrin deposition can be observed in the microvasculature, predominantly in organs such as the lungs, kidneys, gastrointestinal tract, and adrenal glands. Smaller microthrombi may be overlooked in hematoxylin-eosin staining but can be detected using Mallory's phosphotungstic acid hematoxylin staining or electron microscopy. The absence of thrombi in some autopsies may be attributed to postmortem fibrinolysis. Renal examinations may reveal tubular necrosis or severe bilateral cortical necrosis. In a minority of cases, non-embolic endarteritis or pulmonary hyaline membrane disease may be present in the lungs.
bubble_chart Clinical Manifestations
In very mild cases, there may only be laboratory abnormalities. Clinically, based on the acuity of onset and severity of symptoms, it can be divided into acute and chronic types, with the acute type being predominant, manifesting as severe and widespread bleeding. Chronic cases have insidious symptoms, primarily embolism, and the symptoms may be masked by those of the primary disease, not necessarily involving massive bleeding. The symptoms of DIC mainly manifest in four aspects: bleeding, shock, embolism, and hemolysis:
(1) Bleeding: Acute DIC primarily presents as sudden, massive, and widespread bleeding, which may vary depending on the underlying condition. Skin bleeding appears as large patches of ecchymosis or hematomas in one or multiple areas. In obstetric emergencies, there may be massive vaginal bleeding. When it occurs during surgery, the wound may ooze blood continuously or fail to clot. At local injection sites, persistent bleeding from needle punctures may occur. Severe cases may also involve gastrointestinal, pulmonary, or urinary tract bleeding. A rare and special form, fulminant purpura, often occurs with infections, particularly in children with epidemic meningitis. The skin purpura can progress to well-demarcated purple-black skin necrosis and lower limb gangrene, with bleeding mainly concentrated in the lower limbs and buttocks.
(2) Microvascular Embolism Symptoms: These are more noticeable in chronic cases. For example, Trousseau syndrome seen in malignancies may present clinically as migratory thrombophlebitis, while angioma patients may exhibit Kasabach-Merritt syndrome. When thrombi form in the blood vessels of organs, it can lead to ischemic dysfunction or even organ failure, most commonly in the lungs and kidneys. Renal thrombosis often presents with lumbago, hematuria, proteinuria, oliguria, or even uremia and acute renal failure. Pulmonary embolism can cause dyspnea, cyanosis, and respiratory distress syndrome.
(3) Hypotension and Shock: Seen in severe cases, the degree of shock is disproportionate to the amount of bleeding. This is most common in DIC caused by Gram-negative bacterial sepsis and can form a vicious cycle with DIC. It is a sign of severe illness and poor prognosis. Once shock occurs, it can exacerbate DIC and lead to organ dysfunction.
(4) Hemolysis: Often mild and generally not easily noticeable. Microvascular disease-related anemia, in addition to symptoms of anemia and hemolysis, may also present with deformed and fragmented red blood cells in the blood smear.
bubble_chart Auxiliary Examination
Laboratory tests are an important basis for the diagnosis of DIC. Confirmatory tests should directly reflect the activity of thrombin or plasmin, but most of the tests currently used in clinical practice are indirect indicators of their activity. Although many tests are available in this regard, they often lack sufficient sensitivity and specificity. Therefore, clinicians typically combine the results of several tests for diagnosis. Given that DIC is a critical emergency condition, clinical laboratory tests must also be simple, practical, and capable of providing rapid diagnostic guidance for treatment. Consequently, some highly accurate tests are only performed for further diagnosis due to their complex procedures and time-consuming nature. Additionally, test results may vary over time, requiring dynamic observation in conjunction with clinical findings. Laboratory tests are divided into two parts:
Preliminary tests include platelet count, activated partial thromboplastin time (APTT), prothrombin time (PT), and fibrinogen level measurement. If all test results meet the diagnostic criteria, the diagnosis can be confirmed. However, if the results are not entirely consistent, the possibility of false negatives or false positives should be considered, and further tests may be necessary. For example, tests for fibrin monomers can reflect the action of thrombin on fibrinogen, while tests for fibrin(ogen) degradation products (FDPs) can indicate excessive plasmin activity. The D-dimer test is more reliable, as it reflects plasmin-mediated cleavage of cross-linked fibrin.
The diagnostic significance of laboratory test results is summarized as follows:
(I) Test results related to consumptive coagulopathy
1. Thrombocytopenia Generally, the platelet count should be below 100,000/mm3. If the count is >150,000/mm3 (indicating a low likelihood of DIC), the test has limited reference value for patients with pre-existing thrombocytopenia due to underlying diseases.
2. Prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT).
These tests are simple to perform and often show prolongation in the early stages of DIC, with a high positive rate. However, normal results do not exclude a DIC diagnosis. If both PT and APTT are prolonged, it supports the diagnosis of DIC.
3. Fibrinogen level measurement The plasma fibrinogen level should be below 150 mg/dL to be diagnostically significant for DIC. In patients with pre-existing elevated fibrinogen levels, the decrease may not be obvious initially, but follow-up observations may reveal further reductions.
4. Others Prolonged bleeding time, prolonged clotting time, and poor clot retraction may also provide some diagnostic reference value.
(II) Tests for fibrin monomers
1. Plasma protamine sulfate paracoagulation test (3P test): Low concentrations of protamine can induce the polymerization of fibrin monomers. In DIC, the 3P test is positive, with fibrin strands or gel-like formations observed in plasma, whereas it is negative in healthy individuals. False negatives may occur if blood clots during sampling.
2. Ethanol gelation test Adding a 50% ethanol solution can dissociate complexes formed by fibrin monomers and early fibrin degradation products, leading to spontaneous fibrin polymerization and the formation of gel-like clots or fibrin strands. This test has a lower positive rate than the 3P test but higher specificity. Since both methods are relatively simple, they can be performed simultaneously in clinical practice to enhance diagnostic reliability.
(III) Tests for fibrin degradation products
1. Prolonged thrombin time This is caused by reduced fibrinogen levels and/or increased fibrin degradation products. The results may be affected by heparin therapy.
2. Positive red blood cell agglutination inhibition test can be used to detect FDP in the tested serum. When the tested serum is added to red blood cells that have been previously sensitized with anti-fibrinogen, if the serum contains an increased amount of FDP that shares common antigenic clusters with fibrinogen, red blood cell agglutination inhibition occurs.
3. Staphylococcus Agglutination Test Negative Certain coagulase-negative Staphylococcus aureus can be agglutinated by fibrinogen and early fibrin degradation products. If the test turns positive after adding the patient's serum, it indicates the presence of fibrin(ogen) degradation products.
4. Latex Agglutination Test (Fi Test) This test uses latex particles labeled with specific antibodies against fibrinogen, D, and E fragments. If the patient's plasma contains fibrin degradation products, especially D and E fragments, the latex particles will agglutinate.
5. FDP Enzyme-Linked Immunosorbent Assay (ELISA) This method involves an immune reaction between anti-fibrinogen antibodies and antigens in the test sample, followed by the addition of horseradish peroxidase labeling. The resulting color intensity is proportional to the FDP content in the sample.
(IV) Tests Related to Fibrinolytic Activity
1. Euglobulin Lysis Time Measurement The euglobulin fraction in plasma contains fibrinolytic components but lacks plasmin inhibitors. Enhanced fibrinolytic activity shortens the euglobulin lysis time. The normal range is >120 minutes. <70min表示明顯的縮短。
2. Fibrin Plate Lysis Test The patient's plasma sample is added to a fibrin-coated plate. Since the plasma contains a series of fibrinolytic components, the fibrinolytic activity can be calculated by measuring the area of fibrin dissolution after incubation.
(V) Other Diagnostic Tests for DIC
1. Among anticoagulant substances, measurements can be performed for antithrombin III (ATIII) and components of the protein C system.
2. For fibrinolytic activity, the levels of plasminogen antigen, plasminogen activators (tPA), and plasmin (using chromogenic substrate methods) can also be determined.
3. In the detection of fibrin degradation products, D-dimer can be measured, which has higher specificity for DIC diagnosis (commercial kits are available domestically). Elevated plasma FPA levels reflect thrombin generation due to coagulation system activation, helping differentiate primary from secondary fibrinolysis and serving as a monitoring indicator during anticoagulant therapy.
4. Elevated plasma levels of platelet β-thromboglobulin (β-TG) and platelet factor 4 (PF 4 ) indicate platelet activation and enhanced release reactions. Measurements of platelet metabolites such as thromboxane (T x β 2 ) and malondialdehyde (MDA) help assess platelet activation in vivo, contributing to the study of DIC pathogenesis. These tests are less commonly performed in routine clinical practice.
5. Blood Smear Red Blood Cell Morphology Examination In DIC with microangiopathic hemolysis, the blood smear may show fragmented and deformed red blood cells (e.g., helmet cells). A proportion exceeding 2% has diagnostic significance.
DIC occurs on the basis of some primary diseases. Therefore, vigilance should be heightened in diseases where DIC may develop to enable early and definitive diagnosis. Clinically, particular attention should be paid to sudden, unexplained massive or widespread bleeding, coagulation disorders, refractory shock that is difficult to correct, intravascular thrombosis, and organ failure. Acute cases primarily present with massive bleeding, while chronic cases mainly involve thrombosis and may not exhibit obvious massive bleeding. The bleeding in acute DIC must also be distinguished from secondary fibrinolysis and bleeding due to severe liver disease. However, primary fibrinolysis is far less common clinically than DIC. Laboratory findings such as concurrent thrombocytopenia, prolonged PT or/and APTT, and reduced fibrinogen levels—if confirmed by three tests—combined with clinical symptoms, make the diagnosis relatively certain. If only two out of the three criteria are met, potential false positives or false negatives in the tests should be considered, and further testing is necessary. Dynamic observation may also be required when appropriate. Some newer laboratory tests demand advanced technical equipment and specific reagents. The D-dimer test has been clinically applied and offers high reliability in diagnosing DIC.
bubble_chart Treatment Measures
(1) Treatment of disease causes and primary diseases The treatment of the primary disease is a fundamental measure in DIC therapy. For example, active control of infection, removal of uterine contents such as dead fetus or placenta, and antitumor therapy are essential. Failure to control the primary disease is often the main reason for treatment failure.
(2) Supportive therapy Hypoxia, insufficient blood volume, hypotension, shock, and other conditions coexisting with DIC can affect treatment outcomes and should be corrected as much as possible to improve efficacy.
(3) Heparin Opinions on the use of heparin in DIC remain inconsistent. Generally, it is believed that DIC treatment should first target the disease cause. If the disease cause can be quickly eliminated, heparin may not necessarily be used or may be used selectively. For suspected DIC cases or cases with only positive laboratory results, strict indications should be followed. For confirmed DIC cases with predominant embolic symptoms, early use of heparin is recommended to prevent disease progression. Heparin is typically administered in moderate doses, with 50 mg intravenously every 4–6 hours. Alternatively, it can be administered via continuous intravenous infusion at about 10 mg per hour, with a total daily dose of 200–300 mg. The initial dose should not be too large and should be adjusted based on treatment response. Coagulation time should be controlled at 20–30 minutes, and APTT should be maintained at 1–2 1/2 times the normal value. Low-molecular-weight heparin has more stable anticoagulant effects and is considered by some to be superior to standard heparin. Recently, low-dose heparin has been used, administered subcutaneously every 12 hours at 2,500 units per dose. The advantage of low-dose heparin therapy is the absence of bleeding complications and no need for laboratory monitoring. When heparin therapy is effective, plasma fibrinogen levels usually recover within 1–3 days, and FDP decreases. In cases of heparin overdose, protamine sulfate can be administered intravenously for neutralization, along with fresh blood transfusion.
(4) Antiplatelet drugs The commonly used drug is dipyridamole, with an adult dose of 400–800 mg daily, divided into three oral doses, or 100–200 mg diluted in 100 ml of glucose solution for intravenous infusion, repeated every 4–6 hours. Aspirin can also be used at 1.2–1.5 g daily, divided into three oral doses, or both drugs can be combined. These are suitable for mild cases or cases with high suspicion but unconfirmed diagnosis. Additionally, low-molecular-weight dextran (500 ml per intravenous infusion) can reduce blood viscosity and inhibit platelet aggregation, and it can also be combined with dipyridamole.
(5) Antifibrinolytic drugs These are generally used when secondary fibrinolysis is the main bleeding factor. Common drugs include 6-aminocaproic acid, para-aminomethylbenzoic acid, tranexamic acid, or aprotinin. The dose should be reduced after improvement.
(6) Supplementation of platelets or clotting factors If clotting factors are too low, blood transfusion, plasma, or fibrinogen preparations can be administered. Each gram can increase blood concentration by 25–50 mg%. To achieve hemostasis, fibrinogen should be raised above 100 mg/dl. If thrombocytopenia is present, concentrated platelets can be transfused.
(7) Application of ATⅣ concentrate Some administer ATⅣ concentrate intravenously alongside heparin to enhance efficacy, with a dosage of 1,500 units per day (equivalent to the content in 1,500 ml of plasma).





