| disease | Ventricular Septal Defect |
Ventricular septal defect is a common congenital heart malformation. Most cases are single defects, accounting for about 20% of congenital heart diseases; they can also be a component of complex cardiac malformations, such as in tetralogy of Fallot or complete atrioventricular canal. This section focuses solely on simple ventricular septal defects.
bubble_chart Etiology
During the 5th to 7th weeks of embryonic development, muscular septa form from the apex of the ventricle upward and from the bulbar ridge downward. These fuse with the membranous portion of the septum derived from the endocardial cushions of the atrioventricular valves, completing the interventricular septum and fully separating the left and right ventricular cavities. Abnormalities in this developmental process can result in ventricular septal defects (VSDs) in the corresponding areas. These defects are usually single but may occasionally be multiple.
Based on their location, VSDs are generally classified into the following four types (Figure 2): ① **Supracristal VSD**: The defect is adjacent to the pulmonary valve annulus or aortic valve annulus. Larger defects below the aortic valve annulus may cause insufficient support for the right coronary cusp, leading to prolapse of the valve into the defect during diastole and resulting in aortic valve insufficiency. ② **Subcristal VSD**: Also known as perimembranous VSD, this is the most common type. If the defect is large, the unsupported noncoronary cusp above it may prolapse, causing aortic valve insufficiency. ③ **Posterior septal VSD**: Also referred to as an atrioventricular canal-type defect, this is a low membranous defect. Typically large, its right posterior border consists of the septal leaflet and annulus of the tricuspid valve. The atrioventricular conduction bundle passes along the left, posterior, and inferior edges of the defect, requiring caution during surgical repair to avoid misdiagnosis. In rare cases, the defect may be located on the atrial side of the septal leaflet (anatomically, the tricuspid septal leaflet is slightly lower than the mitral septal leaflet), creating communication between the left ventricle and right atrium. ④ **Muscular VSD**: Located in the muscular septum of the right ventricular inflow tract or near the apex, these defects are often multiple and represent a less common type.
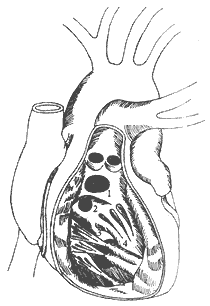
**Figure 2** Classification and locations of ventricular septal defects
⑴ Supracristal defect; ⑵ Subcristal defect; ⑶ Posterior septal defect; ⑷ Muscular defect
The size of a ventricular septal defect can range from a few millimeters to several centimeters. The edges of the defect may be fibrous, muscular, or a combination of both. The diameter of muscular septal defects varies during different phases of the cardiac cycle, becoming smaller during ventricular systole.
bubble_chart Pathological Changes
The pathophysiological effects of ventricular septal defect (VSD) primarily result from the communication between the left and right ventricles, leading to blood shunting and a series of subsequent changes. The magnitude and direction of the shunt depend on the size of the defect and the pressure gradient between the two ventricles, which in turn is determined by the compliance of the right ventricle and the resistance of the pulmonary circulation.
Under normal pulmonary and systemic vascular resistance, the systolic pressure of the left ventricle is significantly higher than that of the right ventricle, with a ratio of approximately 4:1. In VSD, blood flows from the left to the right ventricle through the defect during ventricular systole. In the first few weeks after birth, due to the persistence of some embryonic characteristics in the pulmonary arterioles, pulmonary vascular resistance remains relatively high, resulting in a smaller left-to-right shunt. Subsequently, the shunt volume gradually increases. The increased pulmonary blood flow raises the pressure in the pulmonary veins and left atrium, leading to an accumulation of fluid in the pulmonary interstitium, reduced lung compliance, impaired pulmonary function, and an increased susceptibility to respiratory infections. Therefore, when the shunt volume is large, especially in infants and young children, respiratory distress may occur. Increased respiratory effort raises energy expenditure, and the corresponding reduction in systemic blood flow affects overall growth. The left-to-right shunt at the ventricular level increases the workload of both ventricles. Initially, as pulmonary blood flow increases, total pulmonary resistance can adjust accordingly, so the rise in pulmonary artery pressure is not significant (under normal pulmonary vascular conditions, even a fourfold increase in pulmonary blood flow can be compensated by self-regulation of total pulmonary resistance without a marked change in pulmonary artery pressure). Subsequently, reactive changes such as spasm and contraction occur in the pulmonary arterioles, increasing pulmonary vascular resistance and elevating pulmonary artery pressure. As a result, the pressure in the pulmonary veins and left atrium decreases, improving pulmonary interstitial edema and lung compliance, along with respiratory function and infection susceptibility. Despite this temporary balance and relief phase, the pulmonary arterioles progressively undergo structural changes, including medial hypertrophy, intimal thickening, fibrosis, and luminal narrowing, leading to a continuous rise in pulmonary vascular resistance and severe pulmonary hypertension. With these pathophysiological changes, the left-to-right shunt gradually diminishes, progressing to bidirectional shunting and eventually reversing to a right-to-left shunt. The latter reduces systemic arterial oxygen saturation, causing cyanosis of the lips and extremities, particularly during physical activity—a condition known as Eisenmenger syndrome. At this stage, the left ventricular load decreases, while the right ventricular load further intensifies. The duration of this pathophysiological progression varies with the size of the defect. Large defects may lead to severe pulmonary hypertension by 2–3 years of age, medium-sized defects may delay the onset until around 10 years, and small defects may progress slowly, manifesting only in adulthood, with rare cases remaining asymptomatic for life.
Statistics indicate that approximately 20% of small VSDs may close spontaneously during early childhood. Epidemiological studies show that the prevalence of VSD in infants is about 0.3%, whereas autopsy data from adults reveal a detection rate of only 0.03%, strongly supporting the phenomenon of spontaneous closure. Without surgical intervention, the average lifespan of individuals with VSD is 25–30 years, and life expectancy is significantly shortened once Eisenmenger syndrome develops.bubble_chart Clinical Manifestations
In cases where the defect has a smaller diameter and less shunting, there are generally no obvious symptoms. For larger defects with more significant shunting, symptoms may include developmental delays, palpitations and shortness of breath after activity, recurrent lung infections, and in severe cases, respiratory distress and left heart failure. When grade I to grade II pulmonary hypertension develops and the left-to-right shunting decreases accordingly, conditions such as lung infections may improve, but symptoms like palpitations, shortness of breath, and activity limitations persist or become more pronounced. With grade III pulmonary hypertension and the development of bidirectional or reversed (right-to-left) shunting, cyanosis occurs, known as Eisenmenger syndrome, which worsens with physical exertion or lung infections. Eventually, right heart failure ensues.
During physical examination, patients with larger defects generally exhibit poorer development and are thinner. In advanced-stage cases, cyanosis of the lips and fingers may be observed, and in severe cases, clubbing of fingers (or toes), hepatomegaly, and lower limb edema—signs of right heart failure—may appear. In patients with significant shunting, increased precordial pulsations, bulging of the chest wall, and an enlarged area of cardiac dullness upon percussion may be noted.
Cardiac auscultation: A grade III to IV holosystolic ejection murmur can be heard at the left sternal border in the 3rd to 4th intercostal space (depending on the location of the defect), and a thrill may be palpable in the same area. In cases of elevated pulmonary pressure, an accentuated second heart sound can be heard at the pulmonary valve area. Sometimes, if the defect is partially covered by chordae tendineae, papillary muscles, or abnormal membranous tissue, the murmur may be weaker and the thrill less obvious, but the nature of the ejection murmur can still help in diagnosis. In patients with significant shunting, a diastolic rumbling murmur may also be heard at the apex due to increased blood flow across the mitral valve. In severe pulmonary hypertension with near-equal ventricular pressures, the systolic murmur diminishes or disappears, replaced by a loud second heart sound at the pulmonary valve area or a diastolic murmur of pulmonary regurgitation (Graham Steell murmur). In high ventricular septal defects with aortic valve prolapse and regurgitation, in addition to the systolic murmur, a decrescendo diastolic murmur radiating to the apex may be heard. Due to the short interval between the two murmurs, they may be mistaken for a continuous murmur. Blood pressure measurement may reveal widened pulse pressure and peripheral vascular signs such as femoral artery "pistol-shot" sounds.bubble_chart Auxiliary Examination
Electrocardiogram (ECG) examination: The findings vary depending on the size of the ventricular septal defect (VSD) and the stage of the disease. Small defects may show a normal ECG. In larger defects, the initial stage [first stage] may present with left ventricular hypertension and left ventricular hypertrophy. As pulmonary vascular resistance increases and pulmonary artery pressure rises, combined left and right ventricular hypertrophy gradually appears. In the final stage, right ventricular hypertrophy predominates, and manifestations such as incomplete bundle branch block and myocardial strain may occur.
Echocardiography: This can reveal an echo dropout at the site of the VSD, as well as enlargement of the ventricles, atria, and pulmonary artery trunk. In cases of large high defects combined with aortic valve insufficiency, diastolic valve prolapse may be observed. Color Doppler examination can visualize blood shunting through the defect and, in cases complicated by aortic valve prolapse, diastolic regurgitation. Echocardiography also aids in detecting associated anomalies that may be clinically silent, such as left ventricular outflow tract obstruction or patent ductus arteriosus. In recent years, two-dimensional echocardiography and color Doppler have become the primary diagnostic tools for congenital cardiovascular anomalies, largely replacing cardiac catheterization and angiography.
Chest X-ray examination: Small defects with minimal left-to-right shunting often show no significant changes in the heart, lungs, or great vessels, or may only reveal a slightly prominent pulmonary artery segment or increased pulmonary vascular markings. In larger defects with significant left-to-right shunting and no marked increase in pulmonary vascular resistance, the X-ray may show left and right ventricular enlargement. If the left ventricle is particularly enlarged, it may suggest a large high defect combined with aortic valve insufficiency. The pulmonary artery segment is prominent, and the hilar and pulmonary vascular shadows are enlarged, while the aortic shadow appears relatively small. In advanced cases with markedly increased pulmonary vascular resistance and severe pulmonary hypertension, the cardiac silhouette may paradoxically appear smaller, primarily showing right ventricular enlargement or combined right atrial enlargement. A striking feature is the significant dilation of the pulmonary artery segment and enlarged hilar vascular shadows, while the peripheral pulmonary vascular markings appear normal or even diminished.
Right heart catheterization: Measurement and comparison of oxygen saturation in the right heart chambers. If the right ventricular oxygen content exceeds that of the right atrium by 1.0 volume%, it indicates a left-to-right shunt at the ventricular level. In small defects with minimal shunting, or in cases where the defect is not small but significant pulmonary hypertension reduces left-to-right shunting, the oxygen difference between the right ventricle and right atrium may be less than 1.0 volume%. In such cases, a hydrogen inhalation test can be performed to compare the timing of hydrogen ion curve appearance in various right heart chambers. If the right ventricle shows significantly earlier hydrogen ion appearance than the right atrium, it confirms a left-to-right shunt at the ventricular level. In severe pulmonary hypertension with bidirectional or reversed shunting at the ventricular level, there is no oxygen difference between the right ventricle and right atrium, which can be verified by a concurrent decrease in systemic arterial oxygen saturation. Measurement of right heart chamber pressures (especially continuous pulmonary artery and right ventricular pressure) can reveal right ventricular pressure significantly exceeding pulmonary artery pressure, and the pressure curve characteristics can help identify associated right ventricular outflow tract or pulmonary valve stenosis. The degree of pulmonary hypertension is generally classified by the ratio of pulmonary artery pressure to systemic artery pressure: <40% is grade I, 40–70% is grade II, and >70% is grade III. Calculating pulmonary vascular resistance based on pulmonary artery pressure and cardiac output index aids in determining the timing of surgery and assessing surgical indications and contraindications. The pulmonary-to-systemic blood flow ratio is categorized as low (<1.3), moderate (1.3–2.0), or high (>2.0).
Cardiovascular angiography: Retrograde catheterization to the aortic root with contrast injection can assess whether aortic valve prolapse (insufficiency) is present. Left ventricular angiography can determine the location and size of the VSD and whether it is complicated by left ventricular outflow tract obstruction.
The diagnosis of ventricular septal defect is generally based on medical history, heart murmurs, electrocardiogram, chest X-ray, echocardiography, and color Doppler imaging. Cardiac catheterization and heart blood vessel angiography are only used as supplementary diagnostic measures when necessary.
In addition to understanding the ventricular septal defect itself, it is equally important to determine whether there are any associated malformations, especially the presence of aortic valve prolapse, left ventricular outflow tract stenosis, and patent ductus arteriosus, to avoid adverse consequences caused by misdiagnosis.
bubble_chart Treatment Measures
There are two types of surgical treatments for ventricular septal defects: initial stage [first stage] surgery and intermediate stage [second stage] surgery. The former involves direct defect repair, while the latter entails performing pulmonary artery banding first, followed by elective defect repair at a later date.
Pulmonary artery banding. The method involves encircling the mid-section of the main pulmonary artery with a 3–4 mm wide Teflon strip, tightening the strip, and suturing its ends together. The tightness is adjusted so that the pressure in the distal pulmonary artery is less than 50% of the systemic arterial pressure. This increases right ventricular pressure and reduces left-to-right shunting through the ventricular septal defect, serving as a transitional procedure to avoid the high mortality associated with open-heart repair of large defects during infancy. After 1–2 years, the defect repair is performed electively.
This method was introduced by Müller et al. in 1963 and later widely adopted, based on the clinical observation that patients with simple ventricular septal defects combined with grade II pulmonary stenosis had relatively stable conditions and generally did not develop significant secondary pulmonary vascular changes. In recent years, this procedure has become rare, primarily because the mortality rate of pulmonary artery banding itself is relatively high (around 16%), and when combined with the mortality rate of subsequent defect repair (around 10%), the overall mortality becomes even higher. The tightness of the band is difficult to control, and factors leading to over-looseness or over-tightness during and after surgery are numerous. Over-looseness may necessitate a second banding, while over-tightness can promote reverse shunting through the defect and right heart failure. During the intermediate stage [second stage] surgery, in addition to repairing the ventricular septal defect, the band around the pulmonary artery must be removed, which is technically challenging and risks damaging the pulmonary artery wall. Alternatively, if the pulmonary artery has developed organic stenosis, a lumen-expanding procedure may be required, and some patients may even need reoperation due to residual stenosis. In recent years, with advancements in open-heart surgery for infants, the mortality rate of initial stage [first stage] surgery has become lower than the total mortality rate of intermediate stage [second stage] surgery, and the overall outcomes are better. Therefore, initial stage [first stage] repair is now preferred. Pulmonary artery banding is reserved for a very small number of infants with special conditions, such as multiple fenestrated defects, severe heart failure due to coarctation of the aorta, or other complex intracardiac anomalies that are difficult to correct satisfactorily during infancy.
Ventricular septal defect repair: In principle, once a ventricular septal defect is diagnosed, elective surgery to suture or repair the defect should be performed unless contraindications (see below) exist, to avoid bacterial endocarditis, impaired development and quality of life, or even missing the optimal surgical window.
Small defects may close spontaneously, so surgery can be deferred during infancy. Medium and small defects with minimal pathophysiological impact are ideally repaired before school age. Large defects, which significantly impair cardiopulmonary function—especially in cases where respiratory distress syndrome persists despite aggressive medical treatment—have a high natural mortality rate if left untreated. Due to the early and rapid progression of secondary pulmonary vascular changes, these patients often lose the opportunity for surgery or face excessively high surgical mortality if operated on too late, along with poorer postoperative recovery. Therefore, surgery within 2 years of age is recommended. For high-positioned defects with aortic valve prolapse, early surgery is advised to prevent secondary changes such as structural laxity and elongation of the valve leaflets, as well as worsening aortic regurgitation. Concurrent correction can be performed for coexisting anomalies such as atrial septal defects or patent ductus arteriosus. For patients with coarctation of the aorta, the coarctation can be addressed first, followed by elective ventricular septal defect repair based on hemodynamic status. If left ventricular outflow tract stenosis is present—especially distal to the defect—it must be corrected simultaneously during defect repair. Otherwise, the loss of the decompression "valve" after repair can lead to a sharp increase in left ventricular pressure, resulting in life-threatening left heart failure.
Surgical contraindications: The presence of the following conditions indicates that the disease has progressed too far, and the opportunity for defect repair surgery has been lost. Even if the surgery is forcibly performed and the patient barely survives, there will be no clinical benefit, and there is a risk that the surgery may accelerate deterioration and lead to death. ① Cyanosis occurs at rest or after grade I activity, or clubbing of fingers (toes) is already present. ② The systolic murmur at the defect site is faint or has disappeared, replaced by a diastolic murmur (Graham Steell murmur) caused by pulmonary 2 hyperenhancement or pulmonary stirred pulse valve insufficiency. ③ The stirred pulse oxygen saturation is significantly reduced (<90%); or it is at the normal critical level at rest but drops significantly with minimal activity. ④ Doppler ultrasound shows a bidirectional shunt at the ventricular level, predominantly right-to-left, or a right-to-left (reverse) shunt. ⑤ Right heart catheterization reveals that right ventricular pressure equals or exceeds left ventricular pressure; total pulmonary resistance >10 Wood units (800 dyn·s·cm-5); the ratio of pulmonary to systemic blood flow is <1.2; or the ratio of pulmonary to systemic vascular resistance is >0.75.
Surgical Steps and Techniques: Under general anesthesia with endotracheal intubation, insert a central venous pressure monitoring catheter through subclavian vein puncture, and insert a stirred pulse pressure monitoring catheter through radial stirred pulse puncture. Make a midline incision on the anterior chest, longitudinally saw the sternum, and after incising the pericardium, palpate the right ventricular surface to locate the most prominent systolic ejection tremor as a reference for determining the cardiac incision and identifying the defect site. Place encircling tapes around the superior and inferior vena cava. After heparin injection, insert superior and inferior vena cava drainage cannulas from the right atrium (auricle), and insert a stirred pulse perfusion cannula from the high position of the ascending stirred pulse, then connect them to the cardiopulmonary bypass machine system. After initiating cardiopulmonary bypass (extracorporeal circulation), lower the body temperature via blood flow and maintain systemic temperature at 25–30°C; deep hypothermia may also be used in infants. Clamp the proximal stirred pulse, and inject cardioplegic solution under pressure via a needle (catheter) inserted at its root, while simultaneously perfusing the pericardial cavity with 4°C compound formula Ringer's solution. Place multiple small ice packs around the heart (primarily the ventricles). After cardiac arrest, tighten the superior and inferior vena cava tapes to block venous return. Incise the heart to repair the ventricular septal defect. Begin rewarming via blood flow shortly before completing the intracardiac procedure. During suturing of the cardiac incision, evacuate air from the right heart chambers, and insert a needle at the root of the stirred pulse to remove air from the left heart chambers and stirred pulse. Release the stirred pulse clamp to restore coronary circulation; the heart may resume beating spontaneously. Otherwise, administer electric defibrillation after vigorous ventricular fibrillation. Once the heart beats strongly and the electrocardiogram shows favorable conditions, gradually reduce extracorporeal circulation flow until cardiopulmonary bypass is discontinued. Generally, after releasing the ascending stirred pulse clamp and restoring coronary circulation, maintain high-flow cardiopulmonary bypass for at least 15 additional minutes to maximize recovery of cardiac metabolism and contractile function.
Application of Left Ventricular Decompression Catheter: To prevent left ventricular distension and damage during cardiopulmonary bypass and the initial stage of cardiac resuscitation, a decompression catheter is often inserted into the left atrium via the interatrial groove and passed through the mitral valve into the left ventricle, allowing blood in the cavity to be continuously diverted into the extracorporeal circulation system. The catheter is clamped when cardiac resuscitation is satisfactory and extracorporeal circulation is about to be discontinued. This is particularly suitable for cases with significant cardiac enlargement, poor preoperative cardiac function, those requiring stirred pulse valve prolapse repair, or those complicated by some degree of pulmonary stirred pulse stenosis or abundant pulmonary collateral circulation. It may be omitted for simple small-diameter ventricular septal defect surgeries.
Cardiac Incision: Generally, regardless of the type of ventricular septal defect, the repair can be accomplished via a right ventricular incision. To minimize the impact of the incision on right ventricular function, the incision should be made on the anterior wall of the right ventricular outflow tract. Depending on the distribution of nearby coronary vessels, longitudinal, transverse, or oblique incisions may be used. The incision should be as short as possible while allowing adequate intracardiac manipulation. If extension is necessary, it should preferably extend below the pulmonary stirred pulse annulus and avoid involving the main body of the right ventricle.
To avoid potential impairment of right ventricular function due to the incision, alternative incisions may be selected based on the defect type (anatomical location). A right atrial incision is suitable for membranous and perimembranous defects; the defect is exposed and repaired by retracting the tricuspid septal leaflet. For large membranous defects behind the tricuspid septal leaflet, the base of the leaflet may need to be incised for better exposure. A pulmonary stirred pulse root incision is suitable when the pulmonary stirred pulse trunk and annulus are enlarged; the defect is repaired below the pulmonary stirred pulse valve by retracting the valve. A stirred pulse root incision is suitable for cases requiring concomitant stirred pulse valve prolapse repair or stirred pulse sinus aneurysm repair; the defect can be repaired through the stirred pulse valve orifice. A left ventricular incision is only suitable for multiple apical muscular defects; due to high left ventricular pressure, postoperative bleeding at the incision site is a risk, so caution is advised. In addition to the defect's pathological anatomy, the surgeon's experience and preference are also determining factors in selecting the cardiac incision.
Methods for locating the defect: Based on preoperative examination classification, combined with intraoperative palpation of the right ventricle where the tremor is most pronounced, the defect can be easily identified after opening the cardiac chamber. For small-caliber defects covered by chordae tendineae or membranous tissue, the anesthesiologist can inflate the lungs, and the site where blood gushes out indicates the location of the defect. If the defect cannot be determined by the above methods, diluted methylene blue solution can be injected under pressure via a left ventricular catheter to reveal its location.
Defect repair techniques: Depending on the size and type of the defect, different repair techniques are employed. For defects smaller than 1 cm in diameter, direct suturing is often sufficient. If the edges consist of fibrous tissue, interrupted sutures can be applied directly, with additional mattress sutures reinforced with pledgets if necessary. For small defects with muscular edges, mattress sutures with pledgets are preferable to prevent suture cut-through and ensure surgical efficacy. For defects larger than 1 cm in diameter, it is advisable to use appropriately sized polyester or polytetrafluoroethylene (PTFE) patches to avoid tension-induced dehiscence from direct suturing. During the repair of high-positioned defects, care must be taken to avoid inadvertent injury to the adjacent main stirred pulse valve.
For large membranous defects located behind the tricuspid septal leaflet, the right margin is formed by the septal leaflet annulus, where the conduction bundle runs. Therefore, sutures should be placed at the base of the septal leaflet. The muscular tissue at the to be decocted later angle of the defect should be repaired using mattress sutures with pledgets, placed no less than 5 mm away from the defect edge. The sutures should only penetrate the right ventricular side of the muscular septum to avoid injury to the conduction bundle. The stitch near the septal leaflet should also pass through the base of the leaflet to prevent fistula disease formation after tying. The remaining part can be continuously sutured to the patch, with additional mattress pledget sutures for reinforcement if needed (Figure 1).
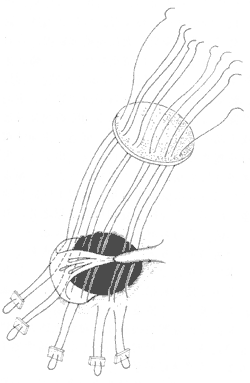
Figure 1: Illustration of the repair method for a large membranous defect behind the tricuspid septal leaflet.
Mattress sutures are placed at the base of the septal leaflet and 5 mm away from the defect edge on the muscular septum to avoid injury to the conduction bundle running along the right, posterior, and inferior margins of the defect.
For high-positioned defects complicated by prolapse of the main stirred pulse valve with significant regurgitation, a low ascending stirred pulse incision is recommended. The elongated and prolapsed valve edge should be folded, and the excess tissue secured to the adjacent stirred pulse wall using mattress pledget sutures, with knots tied externally to ensure equal length with neighboring valve edges and prevent gaps during closure. In rare cases where the prolapsed leaflet exhibits severe secondary degenerative changes and cannot be satisfactorily repaired, valve membrane replacement may be necessary. If the ventricular septal defect cannot be adequately repaired through the stirred pulse incision, an additional right ventricular incision should be made.
For cases with concomitant patent stirred pulse duct, after completing preparations such as extracorporeal circulation cannulation, the duct can be isolated via an extended pericardial incision near the duct and ligated or ligated with pledgets. If compression of the pulmonary stirred pulse causes circulatory instability during the procedure, the repair should be completed promptly under extracorporeal circulation. Alternatively, under extracorporeal circulation, the main pulmonary stirred pulse can be incised, and the ductal orifice sutured or patched from within under low-flow perfusion and head-down positioning. The ventricular septal defect repair can then proceed as usual.
Postoperative management: In addition to standard post-cardiopulmonary bypass care, patients with significant preoperative pulmonary stirred pulse hypertension should remain on ventilator support until the next morning. If weaning from the ventilator fails within 48 hours, tracheostomy should replace endotracheal intubation. Pulmonary stirred pulse hypertension often leads to postoperative circulatory instability, requiring inotropic support. For patients developing third-degree atrioventricular block, pacing efficacy must be ensured. Some cases involve transient conduction bundle injury, with spontaneous recovery within days.
Surgical outcomes depend on disease severity, timing of intervention, surgical precision, and postoperative management. For patients without significant pulmonary stirred pulse hypertension, the mortality rate is below 2%, with favorable prognosis and near-normal recovery. Those with severe preoperative pulmonary vascular complications face higher postoperative respiratory and circulatory complications, increased mortality, and variable recovery depending on the reversibility of pulmonary vascular lesions. Irreversible lesions result in poorer prognosis.
In recent years, due to the accumulation of experience and the improvement of techniques, the incidence of third-degree atrioventricular block has decreased to less than 2%. The residual shunt at the defect site is higher than generally expected, with reports as high as 14–25%. If the residual shunt volume is small and has little hemodynamic impact, clinical follow-up observation may be sufficient. However, before undergoing dental or surgical procedures, potent antibiotics should be administered to prevent bacterial endocarditis. For cases with a larger shunt volume, elective reoperation should be considered.




