| disease | Aortic Valve Stenosis |
Aortic valve stenosis is the most common type of congenital aortic outlet stenosis, accounting for approximately 60% of cases. The primary pathology involves malformation of the aortic valve, resulting in a narrowed valve orifice, and is generally not associated with underdevelopment of the aortic valve annulus.
bubble_chart Pathological Changes
Aortic valve stenosis (Figure 2): This is the most common type of congenital aortic valve stenosis, accounting for approximately 60%. The malformed aortic valve can fuse into a single leaflet, or present as bicuspid, tricuspid, or even quadricuspid, with bicuspid malformation being the most common, accounting for about 70%. The aortic valve shows thickened left and right or anterior and posterior leaflets, with the two commissures fused together, and a small central slit forming the aortic valve orifice. In some cases, the left leaflet is larger and exhibits a thickened ridge, which is a trace of the fusion between the left coronary and non-coronary leaflets. Roberts estimates that about 2% of the population has a bicuspid aortic valve. If the two leaflets do not fuse, it does not cause aortic valve stenosis. However, after the age of 30, due to turbulent blood flow causing valve trauma, the leaflets thicken, become fibrotic, or even calcify, leading to gradual stenosis or insufficiency of the valve orifice. Clinical symptoms may also appear due to concurrent bacterial endocarditis. About 30% of cases have an aortic valve composed of three thickened leaflets, each of similar size, with the edges of the three leaflets fused together, and the central part bulging into the ascending aorta, forming a dome-like structure with the center being the narrow valve orifice. A few patients have a unicuspid aortic valve, resembling an inverted funnel, with a narrow and elongated orifice located centrally or eccentrically on the valve membrane. Sometimes, a faint ridge from the fused commissure can be seen. This type of aortic valve stenosis can present severe symptoms in infancy. Quadricuspid aortic valves are very rare, with the four leaflets possibly being of similar size or one being much smaller than the other three. Quadricuspid aortic valves generally function normally and do not cause stenosis symptoms, often only discovered during autopsy.
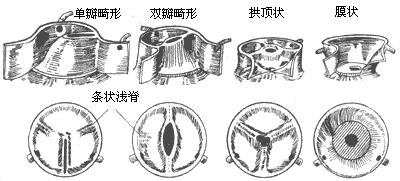
Figure 2 Types of congenital aortic valve stenosis
Pathophysiology: In cases of mild aortic valve stenosis, the impact on cardiac output is minimal, and clinical symptoms are not obvious. When the aortic valve orifice area decreases from the normal 3cm2 to about 1/4 of normal, i.e., 0.75cm2, it significantly affects hemodynamics. To counteract the obstruction, the left ventricle must increase its contractile force and prolong the contraction period, leading to elevated left ventricular pressure, sometimes up to 40kPa (300mmHg), and a pressure gradient between the left ventricle and aorta. In severe cases, the transvalvular systolic pressure gradient can reach 13.3-20kPa (100-150mmHg), causing significant concentric hypertrophy of the left ventricular myocardium without chamber dilation. As left ventricular failure begins, the end-diastolic pressure gradually increases, myocardial contractility weakens, left ventricular systolic pressure decreases, and the left ventricle-aorta transvalvular systolic pressure gradient reduces. Subsequently, left atrial, pulmonary circulation, and right ventricular pressures also increase, leading to left atrial and right ventricular enlargement and myocardial hypertrophy. Blood flow through the narrow valve orifice during left ventricular contraction can cause localized fibrosis and thickening of the ascending aortic wall. Prolonged impact from blood flow can weaken the local vessel wall, leading to post-stenotic dilation of the ascending aorta. Severe left ventricular hypertrophy, prolonged systolic period, and increased left ventricular wall tension can cause subendocardial ischemia, leading to myocardial fibrosis.
bubble_chart Clinical Manifestations
Physical examination: Infants and young children often present with pale skin, rapid breathing, weak pulse, low blood pressure, and cyanosis. Due to reduced cardiac output, the systolic murmur and the transvalvular pressure gradient of the left ventricular aortic pulse are not significant. In children and adolescents, the carotid pulse is strong, the cardiac dullness area is not enlarged, and the apex beat is strong and may be displaced to the left and downward. There is a loud systolic blowing ejection murmur in the aortic valve area, and an early systolic click may be heard. A tremor is often present and can be transmitted to the carotid pulse and apex area. A few patients may also hear a diastolic blowing murmur caused by aortic valve insufficiency, with delayed, weakened, and split second heart sounds in the aortic area. The systolic murmur on the phonocardiogram shows a water calptrop base peel-shaped pattern.
Chest X-ray: In cases with mild stenosis, chest X-ray may show no abnormal signs. Some cases may display dilation of the ascending aorta and left ventricular hypertrophy. In cases of heart failure, cardiac enlargement and pulmonary congestion may be observed. In patients over 25 years old, calcification of the valve may be visible.
Electrocardiogram: In the early stages of the disease and in cases with mild stenosis, there may be no abnormal signs. In cases of grade III stenosis, left ventricular hypertrophy, strain, and left atrial hypertrophy may be evident.
Cardiac catheterization: Increased pressure in the left ventricular cavity and decreased pressure in the aorta are observed. A pressure gradient is present between the left ventricular systolic pressure and the aortic systolic pressure. In grade I stenosis, the pressure gradient at rest does not exceed 5.3 kPa (40 mmHg); in moderate stenosis, the pressure gradient is 5.3-10 kPa (40-75 mmHg); in cases exceeding 10 kPa (75 mmHg), it is classified as grade III stenosis. Measuring cardiac output can help calculate the valve area; in grade III stenosis, the valve area is less than 0.5 cm2/m.
Selective left ventriculography and aortography can reveal thickening of the left ventricular wall, a small left ventricular cavity, and thickening of the valve, which appears dome-shaped. The contrast agent is ejected through the stenotic valve into the aorta. The ascending aorta shows fusiform dilation. Additionally, the activity of the valve, the size of the valve ring, and the presence of aortic valve insufficiency can be assessed.
Echocardiography: Cross-sectional echocardiography can show symmetrical hypertrophy of the interventricular septum and the posterior wall of the left ventricle. The aortic valve leaflets are thickened. The aortic valve closure line is widened during diastole and is perpendicular to the aortic wall. The mobility of the valve leaflets during systole is reduced. The severity of stenosis can be determined by the dome-shaped valve and the diameter of the valve opening.
bubble_chart Treatment Measures
Course of Disease Progression: In cases of congenital aortic valve stenosis where there are no clinical symptoms, normal pulse, no left ventricular hypertrophy or enlargement, and no abnormal signs on the electrocardiogram, approximately 10% of cases begin to show clinical symptoms 10 years after birth. Among these, 20% of cases show transmission from one meridian to the next after 10 years, and 45% of cases develop into grade II or grade III stenosis after 20 years. About 1% of cases develop bacterial endocarditis, which can lead to aortic valve insufficiency. In cases of grade III stenosis with left ventricular hypertrophy and strain on the electrocardiogram, left heart failure may develop and lead to death several years later. The incidence of sudden death is about 1%.
Neonates with congenital aortic valve stenosis presenting with heart failure require emergency surgical treatment. Prostaglandin E1 can be administered short-term before surgery to improve systemic circulation, correct metabolic acidosis, and enhance surgical tolerance. Surgical treatment should be performed in children and adult patients with a left ventricular-aortic systolic pressure gradient exceeding 5.3 kPa (40 mmHg), a resting transvalvular pressure gradient of 5.3-6.6 kPa (40-50 mmHg), or a transvalvular pressure gradient exceeding 9.3-10.7 kPa (70-80 mmHg) even without obvious clinical symptoms.
1. Congenital Aortic Valve Stenosis The goal of surgical treatment is to separate the fused valve commissures, enlarge the valve orifice, and relieve the obstruction to left ventricular outflow without causing valve insufficiency. Alternatively, severely damaged valve tissue can be excised and replaced with an artificial aortic valve.
In 1952, Bailey and in 1954, Brock used a small incision at the left ventricular apex to insert a specially designed aortic valve dilator to separate the fused aortic valve commissures. However, due to incomplete valve dilation and the tendency to cause insufficiency, the results were unsatisfactory, and this method was gradually replaced by direct vision surgery. Starting in 1956, Lewis, Shumway, Swan, and others performed direct vision aortic valve commissurotomy under hypothermic anesthesia, but the limited time for intracardiac surgery under hypothermia resulted in less than ideal outcomes.
In 1958, Spencer et al. reported performing aortic valve commissurotomy under cardiopulmonary bypass, which has since been widely used in clinical practice. In the 1960s, the advent of artificial valves laid the foundation for aortic valve replacement in cases of severe damage or calcification. In recent years, percutaneous balloon aortic valvuloplasty has been developed for patients unable to undergo open-heart surgery, but the number of clinical cases is very small, and long-term efficacy remains to be observed and summarized.
Surgical Procedure: Median sternotomy incision, pericardium is incised, a single venous cannula is inserted into the right atrium, and an arterial cannula is inserted into the ascending aorta. After establishing extracorporeal circulation, the body temperature is lowered to around 30°C, and a venting catheter is inserted into the apex of the left ventricle. The blood flow of the ascending aorta is blocked, and a transverse incision is made approximately 1.5 cm above the annulus at the root of the ascending aorta to expose the stenotic valve. At this point, catheters can be inserted through the openings of the left and right coronary arteries to inject cold cardioplegic solution under pressure, and cold saline is used for local cardiac cooling. Under direct vision, a commissurotomy is performed according to the valve pathology, and the extent of the fused commissure incision should be determined based on the thickness of the commissure and the depth of the adjacent sinus. For well-developed commissures and sinuses, the fused commissure can be incised to within 1 mm of the aortic wall. For underdeveloped commissures and sinuses, only half the length of the fused commissure can be incised. If the commissure shows only traces of fusion, it should not be incised to avoid causing valve insufficiency after incision. A single malformed leaflet can only have one incision. For a bicuspid valve, the fused anterior and posterior commissures between the left and right coronary leaflets and the non-coronary leaflet are incised. For a tricuspid valve with three similarly sized leaflets and well-developed commissures, all three fused commissures can be incised. If the three leaflets vary significantly in size, two fused commissures are incised according to the pathology, converting the aortic valve into a bicuspid type (Figure 1). When incising the fused commissure, atraumatic forceps should be used to retract and fix the leaflets on both sides of the commissure, and a small, sharp scalpel should be used in a sawing motion along the midline of the fused commissure. Scissors should not be used to cut the fused commissure, as the blades can easily slip and deviate from the commissure, cutting the leaflet and causing insufficiency. If the aortic valve leaflets are already fibrotic and thickened or calcified, an artificial aortic valve replacement is required. In pediatric cases with a small aortic annulus, the chosen artificial valve should have a relatively small sewing ring diameter, a larger orifice, and low flow resistance, such as the St. Jude bileaflet valve or a bioprosthetic valve. Sometimes, the aortic root needs to be enlarged.
| (1) Ascending aorta stirred pulse incision | (2) Incision of the commissure | ||
| (3) Commissurotomy site | (4) Ascending aorta stirred pulse incision | (5) Suture of the main stirred pulse incision | |
(I) Extent of commissurotomy for congenital aortic valve membrane stenosis
| (1) "Y" shaped incision of the stenotic area | (2) Patch repair | (3) Completion of patch repair |
(II) Aortic root enlargement for congenital supravalvular aortic stenosis
Figure 1 Surgical management of congenital aortic valve membrane and supravalvular stenosis
Surgical outcomes: Early postoperative mortality is as high as 60% in infants under 1 year of age. In children and adult cases, it drops to below 10%. Preoperative heart function class IV, left ventricular hypoplasia or severe hypertrophy, small left ventricular cavity, and associated congenital mitral valve anomalies or grade III endocardial fibroelastosis increase the risk of surgery.
One year after surgery, cardiac function improved in the majority of cases, and the main stirred pulse pressure increased after exercise. ST segment depression also improved. Approximately 10-30% of cases showed main stirred pulse valve insufficiency, about 50% of cases still had a left ventricle-main stirred pulse transvalvular systolic pressure gradient post-surgery, and about one-third of cases required reoperation 15-20 years after surgery due to gradually worsening residual valve membrane stenosis or recurrence of stenosis.





